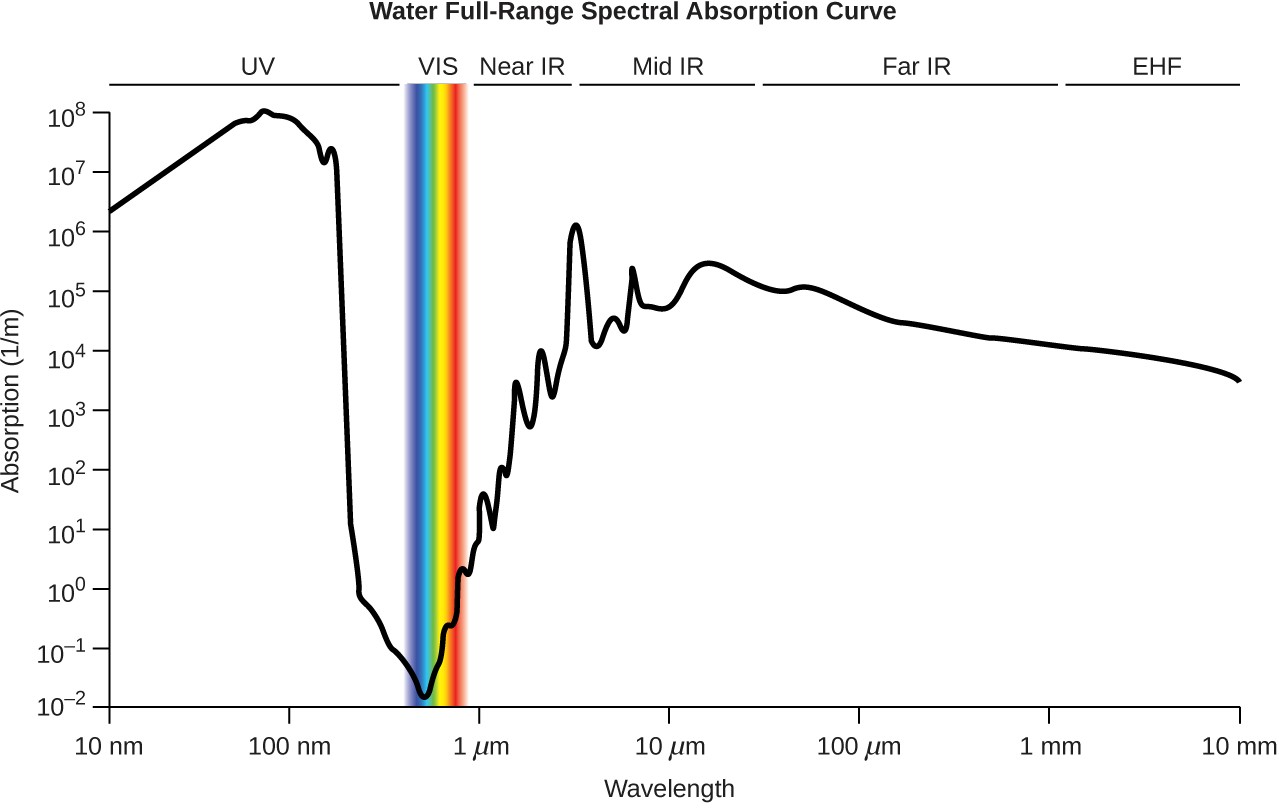
Appendix E – Water Properties
OpenStax
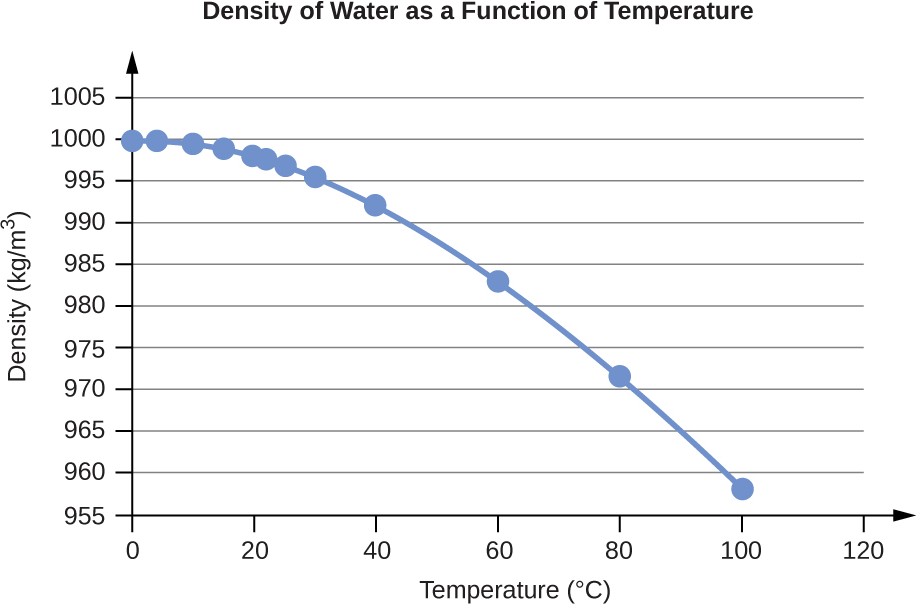
Water Density (g/mL) at Different Temperatures (°C)
|
Temperature |
Density (g/mL) |
|---|---|
|
0 |
0.9998395 |
|
4 |
0.9999720 (density maximum) |
|
10 |
0.9997026 |
|
15 |
0.9991026 |
|
20 |
0.9982071 |
|
22 |
0.9977735 |
|
25 |
0.9970479 |
|
30 |
0.9956502 |
|
40 |
0.9922 |
|
60 |
0.9832 |
|
80 |
0.9718 |
|
100 |
0.9584 |
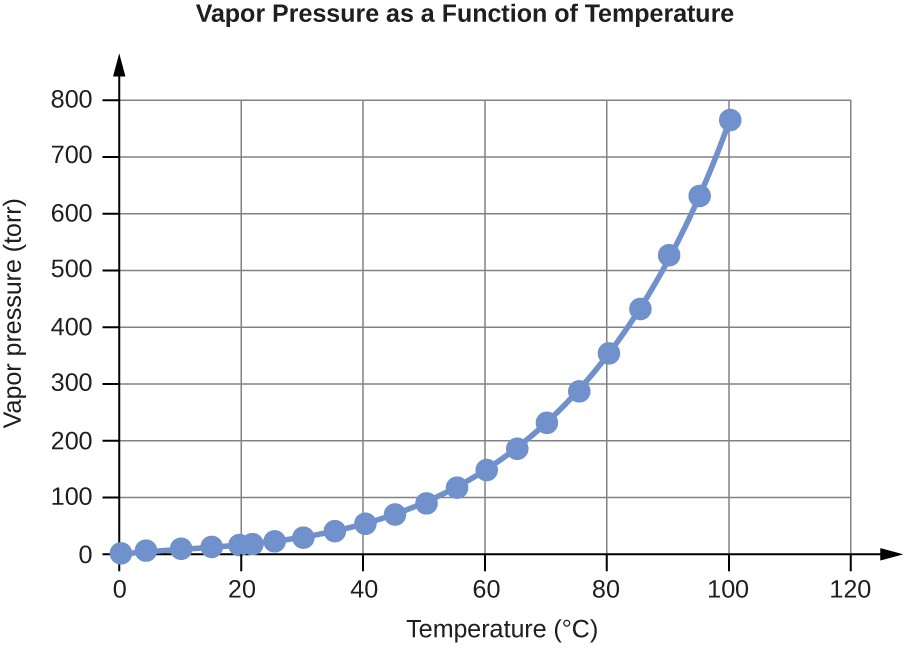
Water Vapor Pressure at Different Temperatures (°C)
|
Temperature |
Vapor Pressure (torr) |
Vapor Pressure (Pa) |
|
0 |
4.6 |
613.2812 |
|
4 |
6.1 |
813.2642 |
|
10 |
9.2 |
1226.562 |
|
15 |
12.8 |
1706.522 |
|
20 |
17.5 |
2333.135 |
|
22 |
19.8 |
2639.776 |
|
25 |
23.8 |
3173.064 |
|
30 |
31.8 |
4239.64 |
|
35 |
42.2 |
5626.188 |
|
40 |
55.3 |
7372.707 |
|
45 |
71.9 |
9585.852 |
|
50 |
92.5 |
12332.29 |
|
55 |
118.0 |
15732 |
|
60 |
149.4 |
19918.31 |
|
65 |
187.5 |
24997.88 |
|
70 |
233.7 |
31157.35 |
|
75 |
289.1 |
38543.39 |
|
80 |
355.1 |
47342.64 |
|
85 |
433.6 |
57808.42 |
|
90 |
525.8 |
70100.71 |
|
95 |
633.9 |
84512.82 |
|
100 |
760.0 |
101324.7 |
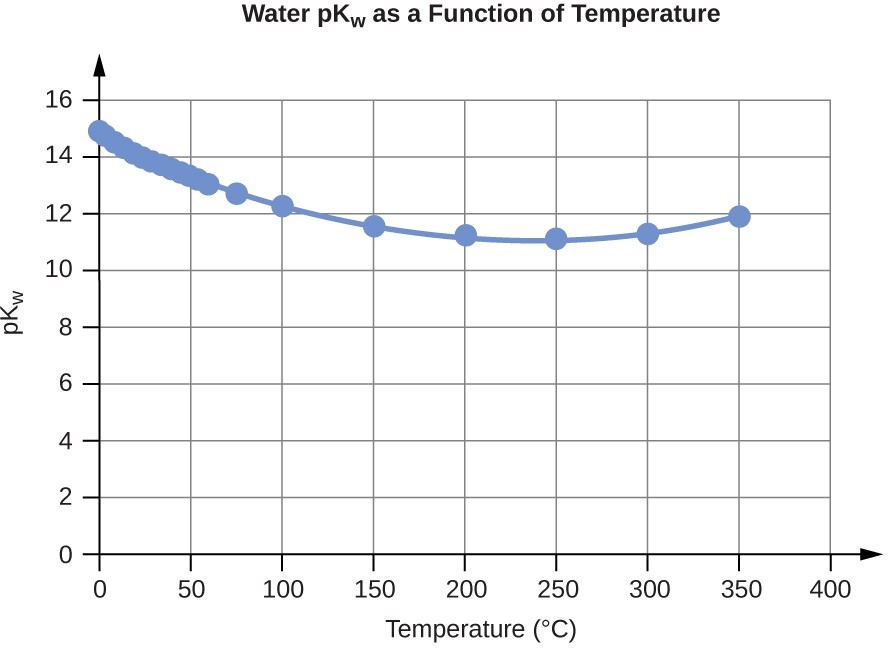
Water Kw and pKw at Different Temperatures (°C)
pKw = –log10(Kw)
|
Temperature |
Kw 10–14 |
pKw |
|---|---|---|
|
0 |
0.112 |
14.95 |
|
5 |
0.182 |
14.74 |
|
10 |
0.288 |
14.54 |
|
15 |
0.465 |
14.33 |
|
20 |
0.671 |
14.17 |
|
25 |
0.991 |
14.00 |
|
30 |
1.432 |
13.84 |
|
35 |
2.042 |
13.69 |
|
40 |
2.851 |
13.55 |
|
45 |
3.917 |
13.41 |
|
50 |
5.297 |
13.28 |
|
55 |
7.080 |
13.15 |
|
60 |
9.311 |
13.03 |
|
75 |
19.95 |
12.70 |
|
100 |
56.23 |
12.25 |
Specific Heat Capacity for Water
|
C°(H2O(l)) = 4.184 J·g-1·°C-1 |
|
C°(H2O(s)) = 1.864 J·K−1·g−1 |
|
C°(H2O(g)) = 2.093 J·K−1·g−1 |
Standard Water Melting and Boiling Temperatures and Enthalpies of the Transitions
|
Temperature (K) |
ΔH (kJ/mol) |
|---|---|
|
Melting – 273.15 |
Boiling – 6.088 |
|
boiling – 373.15 |
40.656 (44.016 at 298 K) |
Water Cryoscopic (Freezing Point Depression) and Ebullioscopic (Boiling Point Elevation) Constants
|
Kf = 1.86°C·kg·mol−1 (cryoscopic constant) |
|
Kb = 0.51°C·kg·mol−1 (ebullioscopic constant) |