Diffusion
Learning Objectives
After completing the lab, the student will be able to:
- Explain or define the term diffusion;
- Explain how temperature affects the rate of diffusion.
Introduction
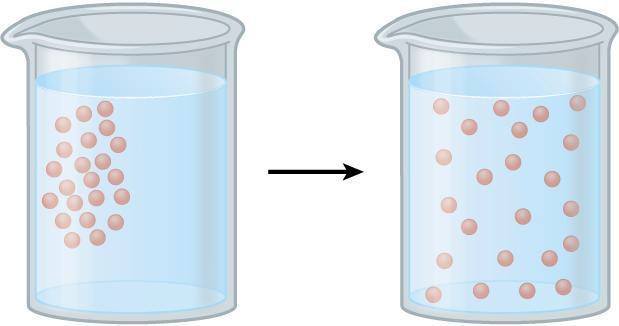
The spontaneous movement of molecules from a higher concentrated area to a less concentrated area is referred to as diffusion. For example, odors spread away from their concentrated source to surrounding areas. Molecules in a gas or liquid are in constant motion because of their kinetic energy. When there are more molecules in one area than another, there are more molecules in position to potentially flow from the concentrated to the sparse area than vice versa, a simple “numbers game.” This inequality drives diffusion (Fig 7.1).
Osmosis is a special case of diffusion: it is water diffusing from a place where it has fewer solutes (dissolved materials) in it, to places where there are more. The driving force is that in the area with more solutes, the number of mobile water molecules is reduced because many are stuck onto molecules of the solute, so effectively less water in these areas is free to move away. Thus there is more opportunity for water flow into those areas than to leave them, and an inequality of water movement results- with a net flow moving from the area with less solute, toward the area with more solute.
In cells, diffusion can move molecules through selectively permeable membranes. In body systems, various constituents such as gases, liquids, and solids are dissolved in water when they flow through the cell membrane from a highly concentrated place to a less concentrated area.
The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will be observing the diffusion of a dye through a beaker of water at different temperatures.
Safety Precautions
- Inform your teacher immediately of any broken glassware, as it could cause injuries.
- Clean up any spilled water or other fluids to prevent other people from slipping.
- Be careful with the dye as it can stain your clothes, and it should not be ingested.
- Wash your hands with soap and water after completion of the activity.
Materials
- Three 250 mL beakers
- Water
- Food coloring
- Thermometer
- Hot plate
- Refrigerator
For this activity, you will work in groups of four.
Procedure
Step 1: Measure 200 mL of room temperature water in a beaker. Do the same for chilled water, and water heated to 50C and 80C.
Step 2: Our goal is to simultaneously place 3 drops of food coloring into the four beakers, and compare their rate of diffusion. Devise a plan that will insure that no beaker gets a “head start” by getting its drops earlier. Warning: Diffusion can happen rapidly in the warmer beakers; find a way to add the three drops in rapid succession and make your observations rapidly. Record your plan in your lab notebook.
Step 3: Record the water temperature in the four beakers. Carry out the Methods you devised in Step 2. Rank order the four beakers in terms of speed of diffusion.
Discussion
In the beakers, the diffusion process eventually stops when the gradient of concentration (difference in concentration from where the drops are inserted, to the surrounding areas) is neutralized. Cells require that gradients that are used to drive molecular movements be maintained, so that diffusion never ends. Speculate: how might the gradient of a molecule between the inside and outside of a cell, be kept strong enough to continually create diffusion of the molecule across the cell’s membrane?

