Semipermeable Membranes
Learning Objectives
After completing the lab, the student will be able to:
- Describe how varying concentrations of solutes and solvents affect the rate of osmosis;
- Describe or explain how temperature affect the rate of osmosis.
Introduction
Membranes encasing cells, and also encasing the nuclei within cells, allow some types of molecules to cross them, and prohibit other types from doing so. An analogy can be found in air filters, such as a face mask used to filter out the coronavirus. The holes in the mask allow tiny air molecules (like N2 and O2, which make up 99% of the air, and which are a mere two atoms in size) to pass, enabling the wearer to breathe, but are too small to allow the much much larger coronavirus pathogen through- it won’t fit through the hole.
Medical dialysis tubing functions similarly with liquids that have dissolved solutes. The holes in the tubing are large enough to allow some molecules to pass, but not others. In the present exercise, they are large enough to allow individual glucose molecules (just 24 atoms in size) to pass, but not starch (variable in size, but containing many linked glucose molecules so much larger in size).
Safety Precautions
- Safety goggles/glasses should be worn when chemicals or solutions are heated.
- Handle all chemicals safely.
- Inform your teacher immediately of any broken glassware, as it could cause injuries.
- Clean up any spilled water or other fluids to prevent other people from slipping.
- Dispose of all chemicals per local regulations.
- Use caution when performing the Benedict’s reaction which involves the use of a hot water bath.
Materials
- Dialysis tubing, two pieces, about 15 cm each long and narrow (pre-soaked)
- Four Dialysis tubing clamps (optional)
- Two 600 mL beaker
- Water
- Glucose (dextrose) powder
- Corn starch powder
- Iodine
- Benedict’s reagent
- Graduated cylinder
- Test tube
- Hot water bath
For this activity, you will work in groups of four.
Procedure
Step 1: Set up a hot water bath at 800 C.
Step 2: Measure 250 mL of room temperature water into each of two 600 ml beakers. Set aside.
Step 3: Make a 5% solution of glucose. Explain in your lab notebook the steps you took to do this.
Step 4: Obtain 1 piece of long (15mm) and narrow dialysis tubing. Seal 1 end of the tube by tying a secure knot or attaching a dialysis tubing clamp to the end of the tubing. Put 10 mL of glucose solution in the tube. Then tie or clamp the other end of the tube. Place the sealed tube into one beaker (Figure 7.2). Continue as you let it sit.
Step 5: Make a 5% solution of corn starch. Explain your lab notebook the steps you took to do this.
Step 6: Repeat the steps used for the glucose, but with the starch. Prepare the dialysis tube in the same fashion, place it in the other 600 ml beaker, and let sit while you work on other parts of the lab.
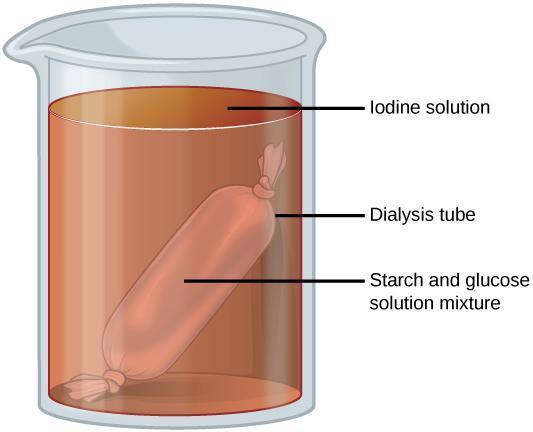
Step 7: Briefly remove the glucose dialysis bag from its beaker and set aside. Pour 2 mL of the water from the beaker into a test tube. Put 10 drops of Benedict’s reagent into the tube (note the initial color of the solution) and place into the hot water bath for 5 minutes. Note whether there is a color change. Place the bag back into the beaker.
Step 8: Put 20 drops of iodine into the water in the starch beaker, leaving the dialysis tube immersed. Allow this to sit for about 10 minutes. Determine whether starch is present in the bag and/or the beaker. Describe in your notebook what you observe.
Step 9: In your notebook, state whether your observations were consistent with the expectation that the dialysis tubing holes were large enough to allow glucose molecules to pass, but too small for starch.
Assessments
In a real cell membrane, more factors than mere pore/size determine which molecules can pass and which cannot. What are some of these other factors and how do they operate? Address the affects of molecular polarity, and function of proteins imbedded in the membrane.

