Osmosis in Living Cells
Learning Objectives
After completing the lab, the student will be able to:
- Describe or explain hypertonic, hypotonic, and isotonic solutions;
- Differentiate between the osmosis mode of action in animal and plant cells.
Introduction
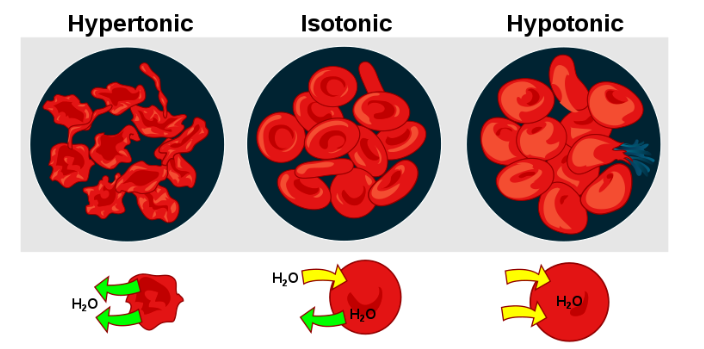
A cell lacking a cell wall is affected greatly by the concentration of dissolved solutes in its surroundings. In a hypertonic solution where the concentration of dissolved solute is higher than in the cell, water will be drawn out of the cell by osmosis. When the cell and surrounding solution have the same solute concentration, they are said to be isotonic. In a hypotonic solution where the concentration of dissolved solute is lower than within the cell, the cell will be under great osmotic pressure from water moving in and can rupture (Figure 7.3).
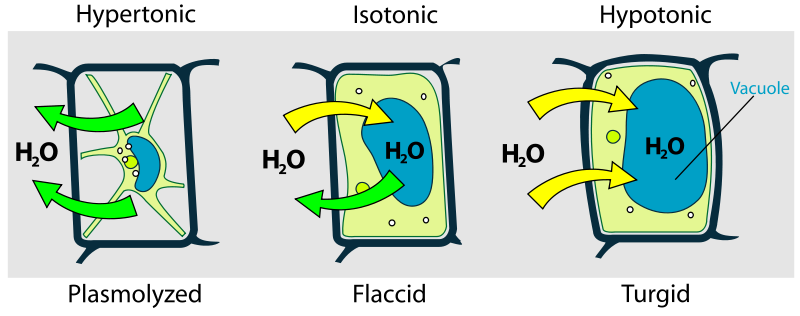
Plants have rigid cell walls composed of cellulose. These cell walls permit for maintenance of cellular integrity when the external environment is hypotonic. In this situation, the water moves into the cell. Without the cell wall, the cell would burst open from the excessive water pressure entering the cell. This state of swelling is referred to as turgid, resulting from turgor pressure (Figure 7.4).
The size and shape of a permeable object will affect the rate of osmosis into or out of it. As an object of any shape shrinks, its inner volume shrinks faster than does its surface area, leaving it with more “skin” in relation to its inner volume. This increases the rate at which its inner state can change by diffusion.
We will use microscopes to observe how cells within Elodea leaves respond to being immersed into solutions with different solute concentrations. Then we will see how immersing cubes of potato into solutions of different solute concentrations, affects their weight (as water enters or leaves the potato in response). Then we will see how changing the shape of the potato cubes speeds or slows this response by altering the amount of surface area.
Safety Precautions
- Be careful handling glass slides; the edges may be sharp.
- Observe proper use of the microscope; avoid handling the electric cord with wet hands.
- Do not use the coarse adjustment knob of the microscope at higher magnifications.
- Inform your teacher immediately of any broken glassware, as it could cause injuries.
- Clean up any spilled water or other fluids to prevent other people from slipping.
- Handle all chemicals safely.
Materials
- Glass slides
- Elodea leaves
- Coverslips
- Distilled water
- Table salt
- Microscope
- Potato cubes
- Electronic balance
- Weigh boat
- Three 50 mL beakers
For this activity, you will work in groups of four.
Procedure
Step 1: Drop a cluster of Elodea leaves in a beaker with distilled water. Let them soak (so that their turgidity reflects its interaction with the distilled water, rather than the tank it was removed from).
Step 2: Make c. 10 ml each of 10% and 30% NaCl solution in small beakers. Describe the steps you took in doing this, in your lab notebook.
Step 3: Obtain a glass slides and 2 cover slips. Place two leaves near opposite ends of the slide. Place a cover slip on one. On the other, place a few drops of 30% saline solution- but not enough to run over and contact the leaf at the other end of the slide. Place a cover slip on it Let these sit for several minutes.
Step 4: Describe and draw any difference you can see between the cells of the Elodea leaves. Do you think water is entering or leaving these cells through osmosis?
Step 5: Predict what might happen to the mass (weight) of a potato cube immersed in the distilled, 10%, and 30% solutions. Write your ideas in your notebook, including the reasons for your predictions.
Step 6: Prepare a table with two columns (“starting mass” and “final mass”), and three rows (the three solutions).
Step 7: Cut three cubes of potato, trying to keep them the same dimensions, and weighing to make sure they are very close to the same mass. Enter these weights in your table, and then immerse the three cubes in the three solutions. Were your predictions correct? If not, propose an explanation for the discrepancy.
Step 8: Cut two cubes that are same mass, but differ in their surface area (make one much flatter, or longer and skinnier, than the cube). Draw them in your lab notebook. Which one do you expect will change its mass the fastest? Immerse them in the same beaker of 30% saline. Might the comparison be rendered uninformative if you let them soak too long? How long do you propose to let them soak? Put these answers in your lab notebook.
Step 9: Prepare another table, similar to the last one, to show the difference in mass of the two pieces of potato. Enter your data. Follow with a sentence concluding if the result was consistent with the idea of greater surface allowing more rapid change to the potato.
Discussion
Have you ever seen a slug hanging out on the steps? Oftentimes, people use salt to get rid of them. What do you think this does to the slug in terms of osmosis?
Includes text and images adapted from Osmosis and Diffusion in Biology OER, a site sponsored by the Ursula Schwerin
Library to select and curate resources for use in General Biology 1 and originally authored and curated by Jeremy Seto,
Department of Biological Sciences – New York City College of Technology. It is licensed CC-BY-NC-SA

