Substrate Specificity of the Enzyme Lactase
Learning Objectives
After completing the lab, the student will be able to:
- Measure enzymatic activity of the enzyme lactase over time and represent it graphically;
- Determine the specificity of the enzyme lactase.
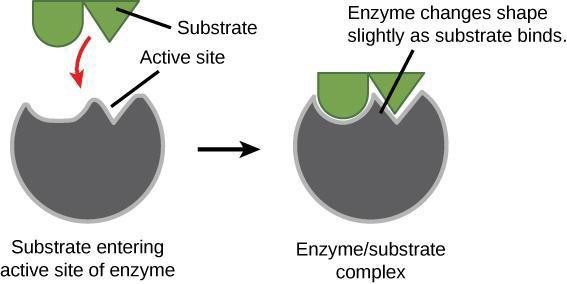
An enzyme’s active site contains side chains of the amino acids that can only bind to certain molecules. For example, if the active site is largely positively charged, then negatively charged substrates will be attracted. Additionally, the three-dimensional shape of the active site within the enzyme is key in determining which substrates will fit into the active site (Figure 7.3). Thus, each enzyme is very specific about what substrate (reactant molecule) it can interact with.
In this experiment, we will determine whether lactase can liberate glucose from the disaccharide sucrose (glucose bound with fructose) as well as it can from lactose (glucose bound with galactose).
Safety Precautions
- Goggles should be worn at all times while in laboratory
- No open toe shoes worn in laboratory
- Measure fluids carefully using graduated cylinders to avoid breakage and spillage.
- Inform your teacher immediately of any broken glassware as it could cause injuries.
- Clean up any spilled fluids to prevent other people from slipping.
Materials
- Graduated cylinder
- Beakers
- Water
- 20 mg/mL lactose solution
- 20 mg/mL sucrose solution
- Lactase
- Stirring rod
- Timer
- Glucose test strips
For this activity, you will work in pairs.
Procedure
Step 1: Prepare 50 ml of sucrose solution, and 50 ml of lactose solution, each with concentration 20 mg/mL. In your lab notebook, describe the masses of sucrose and lactose you used, and ml to which you topped up the beaker with water, to make the solutions. Note that this is lactose (the sugar), not lactase (the enzyme).
Step 2: Prepare two small beakers (small/short enough to dip the glucose strips into), one with 2 mL of lactose solution, and and the other with 2 mL of the sucrose solution. Create a data table to enter your results for each of these test tubes over time, again dipping glucose test strips at 3 min intervals. This time, examine the color at 3 minutes after each dipping (because we are no longer using milk).
Step 3: Add 1 mL of the lactase enzyme solution (the same as used in previous parts of the lab) to each of the two beakers and immediately start timing. When 3 min have elapsed, dip a glucose strip into each beaker, and set them on the table. Continue dipping and assessing strip colors at the times delineated on your table.
Step 4: In your lab notebook, state your results. Did the lactase generate as much glucose from the sucrose as from the lactose?

