Lab 1c Measurement
Learning Objectives
After completing the lab, the student will be able to:
- Measure weight and volume.
Introduction
- Use the following relationships to answer question # 2 below.
- there are 1000 milligrams (mg) in a gram (g)
- there are 8 pints (pt) in a gallon (gal)
- there are 1000 microliters (μl) in a milliliter (ml), and 1000 ml in a liter (L)
- What are the conversions for the following measurements? Record in your lab notebook.
- 50 ml = ____ L
- 20 g = ____ mg
- 700 μl = ____ ml
- 1 gal = ____ pt
- 2000 ml = ____ L
- Compare your answers to #2 with your lab partner or another student in your lab, correct them as needed.
- Density is calculated as mass divided by volume:
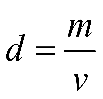
Would you expect the density of a small sample of something to be lower than that of a large sample of the same material, or the other way around, or should they be the same? Record your answer in your notebook, along with an explanation of your thinking.
Safety Precautions
- Inform your teacher immediately of any broken glassware, as it could cause injuries.
- Clean up any spilled fluids to prevent other people from slipping.
- Wash your hands with soap and water after completion of the activity.
Materials
- A balance
- A weighing boat
- A graduated cylinder
- Weightable objects.
For this activity, you will work in pairs.
Procedure
Step 1: Hypothesize/Predict: Predict the rank ordering of the objects you have been given, and water (should be 1 g/ml), in decreasing density. Create a table in your lab notebook with these objects listed in (expected) decreasing order of density, making columns to the right of each for their mass, volume, and density.
Step 2: Devise a plan for measuring the densities of all of these objects. Include instructions regarding how many objects of the same type to use at once, how to measure volume, which graduated cylinder to use, and the detailed steps of carrying out your methods. Record in your lab notebook.
Step 3: Perform your experiment, following the steps and specifications you outlined above. Enter all measurements in the table you created in Step 1.
Step 4: Critical Analysis: Did your methodology work? Was your predicted rank order of density correct? If not, give the details of how it differed. Could you have improved your methods in any fashion, such as to get more accurate or precise measurements? If so, how? Discuss with your partner, and then write your answers in your lab notebook.
Discussion
- Did you feel your density calculation was more accurate for the larger volume objects you measured, or the smaller ones? Why? Record in your lab notebook.

