7 Peer Pointers
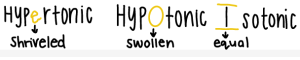
Hypertonic, Hypotonic, and Isotonic solutions: Tonicity refers to the ability for water to move into or out of a cell via Osmosis. Water will always move to areas where there is more solute. In a Hypertonic solution, there is more solute outside of a cell than inside, so the water will move out of the cell, causing it to shrivel. The “e” in Hypertonic looks similar to a shriveled cell. Hypotonic solutions contain more solute inside the cell than outside, so the cell fills with water and swells. The “O” in hypotonic looks like a swollen cell. Isotonic solutions are the golden solutions, because there is an equal amount of solute inside and outside of the cell, so water moves in and out at equal rates. The two lines on the top and bottom of the “I” in Isotonic look like an equal sign.