4 Chapter Four: Principles of Fluids and Electrolytes
Becca Dauzat
Learning Objectives
- Summarize the fundamentals of fluid and electrolyte metabolism and principles of fluid therapy
- Explain the functions of water in the body
- Explain the function of each type of intravenous fluids
- Identify each electrolyte as intracellular or extracellular
- Explain the effects of each electrolyte on the bod
Nursing in Louisiana
Emile Thibodeaux is a 54-year-old commercial crawfisher with a history of Type 2 diabetes, hypertension, and chronic kidney disease. Mr. Thibodeaux presents to the Bayou Regional Medical Center after a long day of running traps in 95oF weather. He reports leg cramps, dizziness, and says he's been drinking "mostly sweet tea and a couple of beers". His skin is warm and dry, mucous membranes are tacky, and his urine is dark amber.
Mr. Thibodeaux is febrile (101.8oF), hypotensive (94/58 mmHg), tachycardic (112 bpm), and tachypneic (22/min).
Foundations of Life
Fluids and electrolytes are the foundation of life, playing essential roles in nearly every physiological process in the human body. Water makes up the majority of our body weight and is more than just a vital component of hydration–it serves as a medium for chemical reactions, a transporter of nutrients, and a regulator of body temperature. Electrolytes, such as sodium, potassium, and calcium, are equally crucial, maintaining electrical neutrality and enabling processes like nerve conductions, muscle contraction, and acid-base balance.
To understand how the body maintains this intricate balance, we must first explore its structure. The body’s water is distributed across various compartments, including the intracellular and extracellular spaces, each with distinct functions and compositions. This distribution is tightly regulated by homeostatic mechanisms, such as the action of hormones like antidiuretic hormone (ADH) and aldosterone which respond to changes in hydration and electrolyte levels.
In this chapter, we will examine the functions of water, the organization of body fluid compartments, and the mechanisms that help maintain equilibrium. We will also discuss the different types of intravenous fluids, examples, and their uses, as well as the unique roles of individual electrolytes. Understanding these concepts is essential for assessing, diagnosing, and managing fluid and electrolyte imbalances.
By the end of this chapter, you will gain insight into how the body maintains its internal environment and learn how to support patients in achieving optimal fluid and electrolyte balance.
Functions of Water
Water is a vital component of life and is essential for several functions in the human body. As a main component of blood and lymph, water acts as a mode of transportation for many essential molecules in the body, such as nutrients, oxygen, wastes, and hormones. It is the primary solvent (add to glossary) in the body, dissolving electrolytes, nutrients, and waste products. This facilitates biochemical reactions and enables cellular metabolism and transportation. Water helps to regulate and maintain body temperature through perspiration (add to glossary) and evaporative cooling mechanisms. When the body heats up, perspiration is produced and evaporates from the skin. This removes heat and prevents overheating. Water is an essential component of bodily fluids that act as lubricants, such as synovial fluid in the joints, cerebrospinal fluid around the brain and spinal cord, and pleural fluid around the lungs. This helps to protect tissues and organs from friction and physical impact. Water helps to maintain cell shape and integrity. It also helps to maintain a balance of fluids and electrolytes, which is critical for normal cell function, nutrient absorption, waste elimination, and other physiological processes. It also plays a major role in buffering systems that regulate the pH within the body, maintaining a stable environment.
Key Takeaways: Functions of Water in the Body
- Transports nutrients, wastes, hormones, enzymes, and blood
- Facilitates cellular metabolism, chemical functioning, digestion, and elimination
- Maintains body temperature
- Lubricates tissues
- Acts as a solvent for substances in the body
Fluid compartments
Total body water (glossary) refers to the total amount of water in the body, which comprises about 60% of an adult’s body weight, but can be affected by different factors, such as age, sex, and body composition. Infants have a high percentage of total body water while older adults have a lower percentage of total body water. Figure 4.2 illustrates the fluid compartments, and Table 4.2 discusses the characteristics of the fluid compartments.
Intracellular (glossary): the intracellular compartment includes fluids within the cell membranes, which accounts for approximately two-thirds of the total body water. The fluid in the intracellular compartment serves as a medium for cellular processes, including metabolism, enzyme activity, and molecular transport.
Extracellular (glossary): the extracellular compartment includes fluids outside of the cell membrane, which accounts for approximately one-third of the total body water. This fluid can be further divided into three compartments.
- The interstitial fluid surrounds cells in tissues and acts as a bridge for nutrient and water exchange between blood and cells.
- The intravascular fluid is contained within the blood vessels and plays an essential role in circulating nutrients, hormones, and waste products throughout the body.
- Transcellular fluids include specialized fluids such as cerebrospinal fluid, pleural fluid, synovial fluid found in joints, pericardial fluid surrounding the heart, and intraocular fluid. These play a critical role in lubricating and protecting organs.
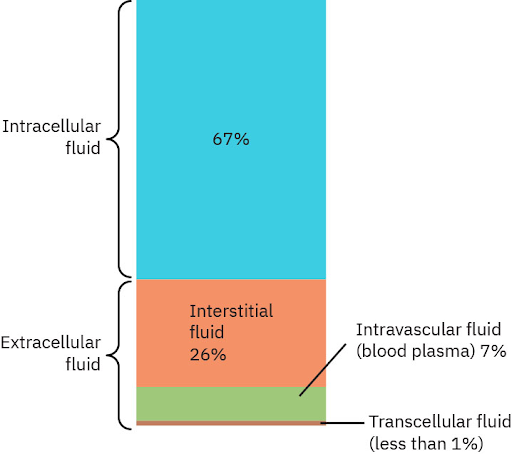
Table 4.2 Characteristics of Body Fluids
| Compartment | Definition | Characteristics |
|---|---|---|
| Intracellular fluid (ICF) | Fluid found inside cells |
|
| Extracellular fluid (ECF) | Fluid found outside of cells
Three subtypes:
|
|
(taken from Fundamentals of Nursing 2e, OpenStax, under CC BY 4.0 license)
Homeostasis Regulation
Homeostasis is essential for optimal functioning of cells and organs within the body. Without homeostasis, the body could not adapt to changes in the environment, and processes like digestion, circulation, and respiration would not be maintained.
Organs Maintaining Homeostasis
There are several organs in the body that work together to regulate fluid balance and maintain homeostasis. Each organ plays a unique role to monitor fluid levels, control electrolyte concentration, and maintain overall fluid and electrolyte equilibrium.
Kidneys:
The kidneys are the primary regulators of fluids and electrolytes. Filtering approximately 180 liters of blood daily, the kidneys selectively reabsorb water and electrolytes to maintain homeostasis while excreting waste and excess substances through urine. The kidneys’ key mechanisms include: the Renin-Angiotensin-Aldosterone System (RAAS), antidiuretic hormone (ADH), and electrolyte regulation.
Lungs:
Through respirations the lungs help to regulate fluid balance. During breathing, water is lost as vapor, contributing to insensible fluid loss. In addition, the lungs help to maintain acid-base balance by controlling carbon dioxide (CO2) levels, influencing blood pH and hydration status.
Skin:
The skin contributes to fluid regulation primarily through sweat production. Not only does sweating help to regulate body temperature, but it also results in the loss of water and electrolytes, including sodium and potassium. This insensible loss through the skin affects fluid balance.
Gastrointestinal Tract:
The gastrointestinal (GI) system helps to maintain fluid balance by absorbing water and electrolytes from consumed foods and fluids. Most water is absorbed in the small intestines, with any excess excreted in the stool. Diarrhea and vomiting can disrupt this balance, leading to significant loss of fluids and electrolytes.
Cardiovascular System:
The cardiovascular system plays a critical role in fluid distribution. The blood vessels transport water, electrolytes, and nutrients to the cells while removing waste products. The heart ensures proper circulation, maintaining adequate perfusion and pressure to support fluid dynamics.
Endocrine system:
Aldosterone, ADH, and atrial natriuretic peptide (ANP) are hormones from the endocrine system that regulate fluid balance. Aldosterone, produced by the adrenal gland, increases sodium and water retention, thereby raising blood volume and pressure. ADH produced by the pituitary gland, reduces water loss through urine when hydration is low. ANP, produced from the heart, promotes sodium excretion to lower blood volume when it is excessive.
Brain:
Osmolality, which is the concentration of solutes in the blood, is monitored by the hypothalamus of the brain. The hypothalamus will stimulate the thirst mechanism when fluid levels are low. It also will signal the release of ADH from the pituitary gland to help conserve water.
Table 4.3 Overview of Organs Responsible for Fluid Balance
| Organ | Contribution to Homeostasis |
|---|---|
| Kidneys |
|
| Lungs |
|
| Skin |
|
| Gastrointestinal Tract |
|
| Cardiovascular System |
|
| Endocrine System |
|
| Brain |
|
Becca Dauzat, CC BY 4.0
Osmosis
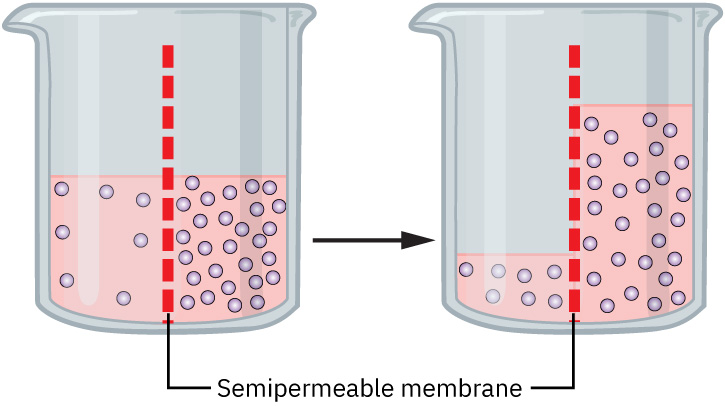
Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Osmosis is driven by osmotic gradients. The force exerted by solutes that draws water across a membrane is known as osmotic pressure. Higher solute concentrations create greater osmotic pressure, pulling water to that area. This process helps balance fluid between the intracellular (inside cells) and extracellular (outside cells) compartments and ensures cells maintain their shape and function.
When there is a change in solute concentrations, osmosis helps to maintain fluid and electrolyte balance. In dehydration, the body loses water and the solute concentrations in the extracellular fluid (ECF) increase. This creates a hypertonic environment. By the process of osmosis, water will move out of cells into the ECF. Excessive shifts can cause cellular dehydration, so the body compensates by triggering thirst and releasing ADH to converse water.
In overhydration, excess water will dilute the extracellular fluid (ECF), creating a hypotonic environment. With osmosis, the water will move into the cells, which can lead to cellular swelling. The kidneys will respond by increasing urine output to remove the excess water.
Thirst Mechanism
The thirst mechanism is a physiological process that helps maintain homeostasis by regulating fluid intake. This process ensures the body has enough water to perform vital functions, maintain blood volume, and balance electrolytes by preventing dehydration, supporting electrolyte balance, and stabilizing blood pressure. It is primarily controlled by the hypothalamus and is activated by signals from the body (i.e., increased serum osmolality, hypovolemia, hypotension) that indicate a need for increased fluid intake. When osmoreceptors in the hypothalamus detect an increase in the osmolality of the blood (i.e., low water levels, high sodium levels), these receptors signal the brain to induce thirst. When baroreceptors in the heart and major blood vessels detect changes in blood pressure and volume (i.e., hypovolemia), the thirst mechanism is activated to replenish fluids.
The pituitary gland will release ADH to work alongside the thirst mechanism to conserve water in the kidneys and encourage fluid intake. Angiotensin II, a hormone produced by the RAAS, also stimulates thirst.
When the thirst mechanism is impaired, it can lead to serious imbalances. Hypodipsia, an inadequate thirst response, may be related to certain neurological conditions or medications or to aging. Polydipsia, excessive thirst, may be related to conditions such as diabetes mellitus or diabetes insipidus.
Antidiuretic Hormone
Antidiuretic hormone (ADH), also known as vasopressin, is produced by the hypothalamus and released by the posterior pituitary gland. ADH plays a critical role in maintaining blood pressure, hydration levels, and electrolyte balance. In response to changing fluid levels in the body, ADH influences the kidneys’ ability to conserve water. By retaining water, ADH indirectly affects electrolyte concentrations, helping to normalize blood osmolality. During dehydration, there is an increase in plasma osmolality, and osmoreceptors in the hypothalamus signal the posterior pituitary gland to release ADH. ADH prevents excessive water loss, ensuring cells and tissues remain hydrated. When there is a decrease in blood volume or pressure, baroreceptors in the heart and major blood vessels signal a release of ADH and the kidneys begin to conserve water and restore the blood volume. In conditions of fluid overload, ADH secretion decreases, allowing the kidneys to excrete excess water and restore balance. As part of the body’s adaptive response, ADH secretion can be stimulated by stress, pain, and certain hormones, such as angiotensin II.
Aldosterone
Aldosterone is a hormone produced by the adrenal cortex. Aldosterone is secreted in response to various stimuli that signal the need to adjust fluid or electrolyte levels. As part of the RAAS, aldosterone conserves sodium and water while excreting potassium. When blood pressure or volume decreases, aldosterone ensures sodium and water are conserved to restore blood volume and pressure. Elevated potassium levels in the blood directly stimulate aldosterone secretion to promote potassium excretion and restore balance. In acid-base imbalance, aldosterone indirectly contributes by influencing hydrogen ion excretion in the kidneys. Stress and release of adrenocorticotropic hormone (ACTH) can also enhance aldosterone secretion as part of the body’s adaptive response.
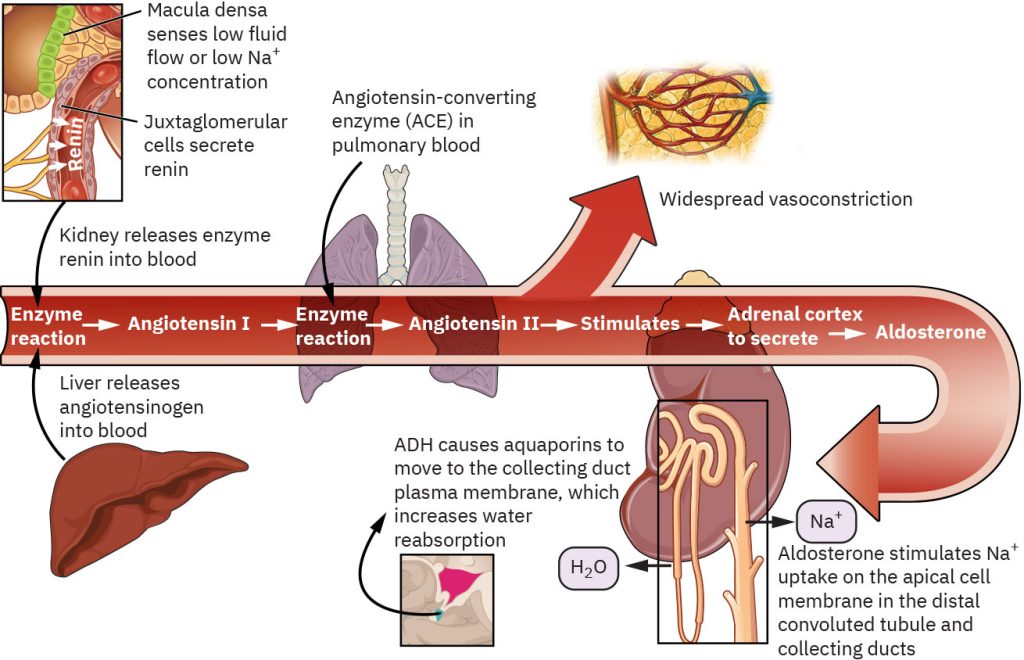
Renin-Angiotensin-Aldosterone System
The RAAS is a vital mechanism for maintaining homeostasis by regulating blood pressure, fluid balance, and electrolyte levels. Its ability to respond to changes in the body ensures stability during times of stress or imbalance. The RAAS is activated by physiological signals and involves several components working together to maintain balance. Specialized cells in the juxtaglomerular apparatus of the kidneys release renin in response to lower blood pressure or volume; a decrease in sodium levels in the blood; and activation by the sympathetic nervous system. Renin converts angiotensinogen (a protein produced by the liver) into angiotensin I. Angiotensin I is converted into angiotensin II by the enzyme angiotensin-converting enzymes (ACE), which is primarily found in the lungs. Angiotensin II promotes vasoconstriction which raises blood pressure by narrowing blood vessels. It stimulates the release of aldosterone from the adrenal cortex and ADH from the posterior pituitary gland. Aldosterone acts on the kidneys to promote sodium reabsorption and potassium excretion. Water is maintained, increasing blood volume and pressure. ADH increases water reabsorption in the kidneys, further contributing to the increase in blood volume.
The RAAS helps the body adapt to changes in fluid and electrolyte balance. In response to decreased blood pressure, the RAAS restores blood pressure by increasing vascular resistance (vasoconstriction) and promoting fluid retention (via aldosterone and ADH). In dehydration, the RAAS minimizes water loss by enhancing sodium and water reabsorption in the kidneys. In sodium imbalances, aldosterone ensures that sodium levels remain stable. Overactivation of the RAAS, as seen in conditions like heart failure or hypertension, can lead to fluid retention, high blood pressure, and strain on the cardiovascular system. Inhibition of the RAAS, as seen with the use of ACE inhibitors or angiotensin receptor blockers, can lead to decreased blood pressure or hyperkalemia. Figure 4.4 demonstrates the RAAS process.
Atrial Natriuretic Peptide
Atrial Natriuretic Peptide (ANP) is a hormone produced and released by the atria of the heart in response to increased blood volume and pressure. ANP is critical in maintaining homeostasis by promoting the excretion of sodium and water, reducing blood volume, and counteracting the effects of the Renin-Angiotensin-Aldosterone System (RAAS). This hormone helps prevent fluid overload and maintains cardiovascular and renal function.
ANP is a key regulator in preventing fluid overload and maintaining electrolyte balance. In response to fluid overload, ANP reduces excess blood volume by increasing diuresis and natriuresis. In blood pressure regulation, ANP helps to lower blood pressure and protect the cardiovascular system by promoting vasodilation and reducing blood volume. In sodium balance, ANP ensures sodium levels are kept within a normal range so as to prevent hypernatremia.
Imbalances in ANP production can contribute to various clinical conditions. Conditions such as heart failure may result in an inadequate release of ANP, contributing to fluid retention, increased blood pressure, and edema. Excessive ANP release can lead to hypotension or electrolyte imbalances due to excessive sodium and water loss.
Types of Fluids
Isotonic Fluids
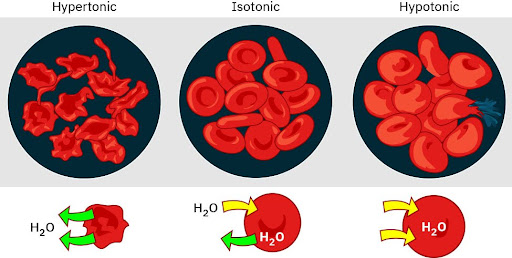
Isotonic intravenous fluids have a similar concentration of solutes as blood plasma. Because the osmotic concentration or osmolarity is equivalent to that of the body, isotonic fluids neither pull water into cells nor push water out of cells. This is particularly useful for maintaining fluid balance in the intravascular space without causing a significant shift in fluid between the intracellular and extracellular compartments. Figure 4.5 demonstrates the effects of isotonic fluids on the cell.
Isotonic fluids are essential in balancing fluid volume and maintaining cellular homeostasis. They can be used to restore intravascular volume in cases of hypovolemia, and to maintain blood pressure and cardiac output. They are also used as a method for delivering medications and electrolytes.
Different isotonic fluids contain varying electrolytes; the choice depends on the patient’s needs. Examples of isotonic intravenous fluids include 0.9% Sodium Chloride (normal saline), Lactated Ringer’s Solution, and 5% Dextrose in Water. Normal saline is used for fluid resuscitation in dehydration and blood loss and for restoring extracellular fluid volume. Lactated Ringer’s Solution is used for replacing fluids and electrolytes, especially after surgery or trauma and for correcting acidosis. 5% Dextrose in Water is initially isotonic but becomes hypotonic as the dextrose is metabolized and is used for providing free water for hydration.
Important Considerations for Isotonic Fluids
Excessive administration of isotonic fluids can lead to fluid overload, potentially causing complications such as edema or pulmonary congestion. Patients with kidney disease, heart failure, or liver problems may require careful monitoring when receiving isotonic fluids.
Hypotonic Fluids
Hypotonic intravenous fluids are solutions with a lower concentration of solutes compared to the blood plasma. Because the osmolarity is less than that of the body, water shifts from the extracellular space into the intracellular space due to osmosis. This type of fluid is used to rehydrate cells in conditions of intracellular dehydration, such as hypernatremia and diabetic ketoacidosis, and to maintain daily fluid requirements when sodium levels need to be minimized. Figure 4.5 demonstrates the effects of hypotonic fluids on the cell.
Examples of hypotonic solutions include 0.45% Sodium Chloride (Half Normal Saline) and 5% Dextrose in Water after the dextrose is metabolized. 0.45% Sodium Chloride is typically used for treating cellular dehydration. 5% Dextrose in Water is used to provide free water for hydration and to support cellular functioning.
Examples of hypotonic solutions include 0.45% Sodium Chloride (Half Normal Saline) and 5% Dextrose in Water after the dextrose is metabolized. 0.45% Sodium Chloride is typically used for treating cellular dehydration. 5% Dextrose in Water is used to provide free water for hydration and to support cellular functioning.
Important Considerations for Hypotonic Fluids
Hypotonic solutions are valuable for rehydrating cells but must be used cautiously to prevent complications related to fluid shifts and electrolyte disturbances. Dilution of serum sodium and other electrolytes may occur, leading to conditions like hyponatremia. Because of the shift of water into the cells, excessive administration can lead to cellular overhydration. In the brain, this may result in cerebral edema, a potentially life-threatening condition. These fluids should be avoided in patients with increased intracranial pressure, burns, and hypovolemic shock. Hypotonic solutions should be used cautiously and under close monitoring.
Hypertonic Fluids
Hypertonic intravenous fluids are solutions with a higher concentration of solutes compared to the blood plasma. Because the osmolarity exceeds that of the body, water shifts from the intracellular space into the extracellular space due to osmosis. This type of fluid is used to reduce cellular swelling and expand intravascular volume, such as in cases of severe hyponatremia, cerebral edema, and hypovolemic shock. Figure 4.5 demonstrates the effects of hypertonic fluids on the cell.
Examples of hypertonic solutions include 3% Sodium Chloride (Hypertonic Saline), 5% Sodium Chloride, Dextrose 10% in Water, and Dextrose 5% in 0.45% or 0.9% Sodium Chloride. 3% Sodium Chloride and 5% Sodium Chloride are used for treating severe hyponatremia and reducing cerebral edema, with 5% Sodium Chloride being used less often due to its high osmolarity. Dextrose 10% in Water is used for providing calories in parenteral solutions and treating hypoglycemia. Dextrose 5% in 0.45% or 0.9 Sodium Chloride is used to manage fluids and electrolytes balances when calories are needed.
Important Considerations for Hypertonic Fluids
Hypertonic fluids are important in medical care but they require careful administration to prevent serious side effects while achieving therapeutic goals. Patients receiving hypertonic fluids require frequent monitoring of electrolytes, fluid status, and neurological function. The use of these fluids could lead to pulmonary edema, cellular dehydration, and hypernatremia. They can be irritating to peripheral veins and are often administered via a central venous catheter. These fluids must be infused slowly and carefully to avoid rapid shifts in fluid balance and should only be used where the benefits outweigh the risks.
Table 4. 4
| Characteristic | Isotonic Fluid | Hypotonic Fluid | Hypertonic Fluid |
|---|---|---|---|
| Tonicity | Contains the same number of solutes compared with blood | Contains fewer solutes than blood contains | Contains more solutes than blood contains |
| Fluid movement | No net movement of fluid | Fluid moves into cells (can cause cellular swelling) | Fluid moves out of cells (can cause cellular shrinking) |
| Clinical situations used | Fluid replacement in states such as:
|
|
|
| Nursing considerations |
|
|
|
| Examples |
|
|
|
Types of IV Fluids (Modifications of work by B. Dauzat from Anatomy and Physiology 2e. Rice University, OpenStax, under CC BY 4.0 license)
Electrolytes
Sodium
Sodium is a major extracellular electrolyte. The normal serum value is 136-145 mEq/L. Sodium plays a vital role in maintaining fluid balance, nerve impulse transmission, and muscle function. It is the most abundant cation (need to find expert definition for glossary) in extracellular fluid and is critical for regulating water distribution and supporting numerous physiological processes. Sodium levels are regulated by the RAAS, ADH, ANP, and dietary intake and excretion.
Proper sodium levels prevent dehydration and fluid overload. By regulating water distribution between intracellular and extracellular compartments through osmosis, it is the primary determinant of extracellular fluid osmolality. Sodium is critical for generating and transmitting electrical signals in the nerves. During an action potential, sodium ions move into nerve cells, creating the electrical impulses needed for electrical communication. Sodium works in conjunction with potassium and calcium to facilitate depolarization and repolarization of muscle cells, particularly cardiac and skeletal muscles. Sodium helps to maintain acid-base balance by aiding the kidneys in exchanging hydrogen ions.
Potassium
Potassium is a major intracellular electrolyte. The normal serum value is 3.5-5.2 mEq/L. Potassium plays a key role in maintaining cellular function, nerve signaling, and muscle contraction. It is the primary intracellular cation, and is vital for maintaining the body’s normal electrical and chemical balance. Potassium is regulated by dietary intake, renal excretion, and shifts between the intracellular and extracellular compartments.
Potassium is critical for establishing and maintaining the rest membrane potential of cells, particularly in the nerves and muscles, which is essential for the generation of action potentials enabling nerve signaling and muscle contractions. It works closely with sodium and calcium to ensure proper nerve conduction and muscle activity by facilitating the repolarization phase of nerve and muscle cells during an action potential. Potassium assists in maintaining the body’s acid-base equilibrium by exchanging with hydrogen ions in the kidneys, and it also influences enzyme activity, protein synthesis, and carbohydrate metabolism--processes essential for energy production and cell function.
Calcium
Calcium is a major extracellular electrolyte. The normal serum value is 8.8- 10.4 mg/dL. Calcium is vital for bone health, muscle function, and nerve transmission. It is the most abundant mineral in the body, with 99% stored in bones and teeth. The other 1% circulates in the blood and other tissues. Calcium is regulated by the parathyroid hormone, calcitonin, and vitamin D.
Calcium provides structural strength to bones and teeth, supporting growth, maintenance, and repair. The bone acts as a reservoir for calcium, releasing it into the bloodstream as needed to maintain balance. It is essential for the contraction of skeletal, cardiac, and smoother muscle. During the contraction of a muscle, calcium ions are released within muscle cells, triggering the interaction between actin and myosin filaments. Calcium supports the generation and propagation of nerve impulses by facilitating the release of neurotransmitters at synapses. It plays a critical role in the coagulation cascade by activating clotting factors, aiding in the formation of blood clots. Calcium serves as a signaling molecule in various cellular processes, including hormone secretion, enzyme activation, and cell division. Calcium contributes to maintaining the balance of the blood’s pH by buffering acidic or basic shifts.
Magnesium
Magnesium is a major intracellular electrolyte. The normal serum value is 1.8-2.6mg/dL. Magnesium is vital for numerous cellular functions, enzyme activity, and neuromuscular processes. About 60% of the body’s magnesium is stored in the bones, while the rest is distributed in muscles, soft tissues, and extracellular fluid. Magnesium is regulated by dietary intake and renal excretion.
Magnesium supports metabolic processes such as glycolysis and adenosine triphosphate (ATP) formation and is involved in energy production, protein synthesis, and deoxyribonucleic acid (DNA) replication. Magnesium regulates nerve impulse transmission and muscle contraction by stabilizing cell membranes and controlling the movements of ions into and out of the cells. It helps maintain normal heart rhythm by balancing calcium and potassium levels within cardiac cells and preventing hyperexcitability in cardiac tissues and contributes to bone formation by supporting calcium uptake and regulating bone density. Magnesium enhances insulin action, promoting glucose uptake by cells and helping to regulate blood sugar levels. Magnesium has a relaxing effect on blood vessels, supporting normal blood pressure by reducing vascular resistance.
Chloride
Chloride is a major extracellular electrolyte. The normal serum value is 96-106 mEq/L. Chloride works closely with sodium and potassium to maintain fluid balance, acid-base regulation, and overall homeostasis. It plays an important role in a variety of physiological processes, particularly in maintaining the electrical neutrality of bodily fluids. Chloride is regulated by dietary sources and renal excretion.
Chloride helps to maintain osmotic pressure and fluid balance, along with sodium, by regulating the movement of water between intracellular and extracellular compartments. Chloride helps to maintain blood pH within the normal range of 7.35-7.45 by exchanging with bicarbonate ions in red blood cells (known as the chloride shift). It aids in digestion and provides an acidic gastric environment by producing hydrochloric acid in the stomach. Chloride supports nerve signaling and muscle contraction, helping maintain electrical neutrality across cell membranes.
Phosphate
Phosphate is a major intracellular electrolyte. The normal serum value is 2.7-4.5 mg/dL. Phosphate plays a central role in energy production, cellular function, and bone health. Approximately 85% of phosphate is stored in the bones and teeth, with the remaining 15% being found in soft tissues and extracellular fluid. Phosphate is regulated by dietary intake, renal regulation, parathyroid hormone, and vitamin D.
Phosphate is a critical component of ATP, the primary energy of cells, and is essential for energy storage and transfer within the body. Phosphate combines with calcium to play a vital role in bone growth, maintenance, and repair. It helps to maintain acid-base balance by neutralizing excess acids and bases and is involved in DNA and ribonucleic acid (RNA) synthesis, supporting genetic material replication and protein synthesis. Phosphate activates many enzymes involved in metabolic processes, including carbohydrate metabolism, and also assists red blood cells in releasing oxygen to tissues.
Table 4.5
| Electrolyte | Major Fluid Compartment | Normal Range | Function in the Body | Regulation |
|---|---|---|---|---|
| Sodium | Extracellular | 136-145 mEq/L |
|
|
| Potassium | Intracellular | 3.5-5.2 mEq/L |
|
|
| Calcium | Extracellular | 8.8-10.4 mg/dL |
|
|
| Magnesium | Intracellular | 1.8-2.6 mg/dL |
|
|
| Chloride | Extracellular | 96-106 mEq/L |
|
|
| Phosphate | Intracellular | 2.7-4.5 mg/dL |
|
|
Overview of electrolytes within the Body. Dauzat, CC BY 4.0
Conclusion
The balance of fluids and electrolytes is essential to health, supporting critical bodily functions like nutrient transport, waste elimination, nerve signaling, and muscle activity. Understanding how water is distributed across body fluid compartments, the mechanisms that regulate this balance, and the specific roles of key electrolytes equips nurses with the tools needed to assess and manage patients effectively.
This chapter has explored the functions of water, the structure and significance of body fluid compartments, and the complex homeostatic processes that maintain equilibrium. We’ve also examined the characteristics and uses of different types of fluids, along with the vital roles of electrolytes such as sodium, potassium, and chloride. Together, these elements form the framework for interpreting and addressing fluid and electrolyte imbalances.
Louisiana Content Wrap Up
Mr. Thibodeaux’s sodium level is 128 mEq/L and his potassium level is 3.1 mEq/L. The nurse identifies signs of fluid volume deficit and electrolyte imbalance and initiates IV therapy to restore hydration and correct his electrolyte imbalances.
Questions to ponder:
- Which IV solution would be most appropriate for initial fluid replacement?
- How might Mr. Thibodeaux’s diabetes and kidney disease affect fluid and electrolyte management?
- What nursing assessments are most important to monitor for signs of improving or worsening electrolyte imbalance?
- How could patient education prevent recurrence of this condition during future fishing trips?
Key Takeaways
- Some cohort members prepared this as a bulleted list
Review Questions
References:
“Outline key considerations for a fluids and electrolytes chapter of a nursing textbook”. (OpenAI, 2025). OpenAI. (2024). ChatGPT (Dec 11 version) [Large language model]. https://chat.openai.com/chat
Bowen, C., Carey, B., Palozie, J., & Reinholdt, M. (2024). Medical-surgical nursing. OpenStax. https://openstax.org/details/books/medical-surgical-nursing
Ernstmeyer, K., & Christman, E. (2024). Nursing fundamentals 2e. Open RN WisTech Open. https://wtcs.pressbooks.pub/nursingfundamentals/
Attribution
Original text by Becca Dauzat, RN, MSN, CNE with outline from ChatGPT with additional text by Bowen, C., Carey, B., Palozie, J., Reinholdt, M., Ernstmeyer, K., & Christman, E. CC BY 4.0
Chapter opener: When prompted with “Please provide a South Louisiana-themed patient scenario that would be found in a textbook related to intravenous therapy for front-line healthcare professionals. This would be introducing the chapter on fluids and electrolytes.” the ChatGPT-generated text provided the above text. (OpenAI, 2025)
This process involves various biological mechanisms that detect changes, trigger responses, and restore balance. Examples of things that homeostasis controls include body temperature, chemical energy, pH levels, oxygen levels, blood pressure, and blood sugar.
Aldosterone (ALD) is a hormone your adrenal glands release that helps regulate blood pressure by managing the levels of sodium and potassium in your blood.
the fluid that circulates in the heart, arteries, capillaries, and veins of a vertebrate animal carrying nourishment and oxygen to and bringing away waste products from all parts of the body
a usually clear coagulable fluid that passes from intercellular spaces of body tissue into the lymphatic vessels, is discharged into the blood by way of the thoracic duct, and resembles blood plasma in containing white blood cells and especially lymphocytes but normally few red blood cells and no platelets
Hormones are chemical messengers that coordinate different functions in your body. Several glands, organs and tissues make and release hormones, many of which make up your endocrine system.
1. The state of equilibrium (balance between opposing pressures) in the body with respect to various functions and to the chemical compositions of the fluids and tissues.
2. The processes through which such bodily equilibrium is maintained.
Hypertonic refers to a solution with higher osmotic pressure than another solution. In other words, a hypertonic solution is one in which there is a greater concentration or number of solute particles outside a membrane than there are inside it.
A hypotonic solution is defined as a solution that has a lower solute concentration compared to another solution, such as the cytoplasm inside a cell. This difference in concentration causes water to flow into the cell. In essence, a hypotonic solution results in a net movement of water into the cell due to osmosis.
An osmoreceptor is a sensory receptor primarily found in the hypothalamus of most homeothermic organisms that detects changes in osmotic pressure. Osmoreceptors can be found in several structures, including two of the circumventricular organs – the vascular organ of the lamina terminalis, and the subfornical organ. They contribute to osmoregulation, controlling fluid balance in the body. Osmoreceptors are also found in the kidneys where they also modulate osmolality.
Hypovolemia, also known as volume depletion or volume contraction, is a state of abnormally low extracellular fluid in the body. This may be due to either a loss of both salt and water or a decrease in blood volume. Hypovolemia refers to the loss of extracellular fluid and should not be confused with dehydration.
Angiotensin II (Ang II) is a medication that is used to treat hypotension resulting from septic shock or other distributive shock. It is a synthetic vasoconstrictor peptide that is identical to human hormone angiotensin II[3] and is marketed under the brand name Giapreza.
Hypodipsia, also known as adipsia, is a symptom of inappropriately decreased or absent feelings of thirst.[1][2] It involves an increased osmolality or concentration of solute in the urine, which stimulates secretion of antidiuretic hormone (ADH) from the hypothalamus to the kidneys. This causes the person to retain water and ultimately become unable to feel thirst.
Diabetes, also known as diabetes mellitus, is a group of common endocrine diseases characterized by sustained high blood sugar levels
Diabetes insipidus ("tasteless" diabetes, as opposed to diabetes mellitus) can also cause polydipsia.
The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones
Adrenocorticotropic hormone (ACTH; also adrenocorticotropin, corticotropin) is a polypeptide tropic hormone produced by and secreted by the anterior pituitary gland. It is also used as a medication and diagnostic agent.
The juxtaglomerular apparatus (also known as the juxtaglomerular complex) is a structure in the kidney that regulates the function of each nephron, the functional units of the kidney. The juxtaglomerular apparatus is named because it is next to (juxta-) the glomerulus.
Renin, also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin-angiotensin-aldosterone system (RAAS) that increases the volume of extracellular fluid (blood plasma, lymph, and interstitial fluid) and causes arterial vasoconstriction. Thus, it increases the body's mean arterial blood pressure.
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles.
Heart failure (HF), also known as congestive heart failure (CHF), is a syndrome caused by an impairment in the heart's ability to fill with and pump blood.
Hypertension, also known as high blood pressure, is a long-term medical condition in which the blood pressure in the arteries is persistently elevated.[11] High blood pressure usually does not cause symptoms itself.[1] It is, however, a major risk factor for stroke, coronary artery disease, heart failure, atrial fibrillation, peripheral arterial disease, vision loss, chronic kidney disease, and dementia. Hypertension is a major cause of premature death worldwide
Angiotensin-converting-enzyme inhibitors (ACE inhibitors) are a class of medication used primarily for the treatment of high blood pressure and heart failure.
Angiotensin receptor blockers, also called angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system.
Hyperkalemia is an elevated level of potassium (K+) in the blood.
Atria (heart) (singular: atrium), an anatomical structure of the heart
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood.
Edema (American English), also spelled oedema (British English), and also known as fluid retention, swelling, dropsy and hydropsy, is the build-up of fluid in the body's tissue.
Hypotension, also known as low blood pressure, is a cardiovascular condition characterized by abnormally reduced blood pressure.
A solution is isotonic when its effective osmole concentration is the same as that of another solution. In this case the cell neither swells nor shrinks because there is no concentration gradient to induce the diffusion of large amounts of water across the cell membrane.
Osmotic concentration, formerly known as osmolarity,[1] is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar").
Fluid replacement or fluid resuscitation is the medical practice of replenishing bodily fluid lost through sweating, bleeding, fluid shifts or other pathologic processes.
Acidosis is a biological process producing hydrogen ions and increasing their concentration in blood or body fluids. pH is the negative log of hydrogen ion concentration and so it is decreased by a process of acidosis.
Ketoacidosis is a metabolic state of acidosis mostly due to uncontrolled diabetes mellitus, where there is a deficiency of adequate insulin to use sugar or glucose as a source of energy
Serum is the fluid and solvent component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed.
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific cell rapidly rises and falls
Depolarization or hypopolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell compared to the outside.
the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value.
Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates serum calcium and phosphate through its actions on the bone, kidneys, and small intestine. PTH increases serum calcium levels and is counteracted by calcitonin.
Calcitonin is a 32 amino acid peptide hormone secreted by parafollicular cells (also known as C cells) of the thyroid. It acts to reduce blood calcium (Ca2+), opposing the effects of parathyroid hormone (PTH).
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.
Myosins are a family of motor proteins (though most often protein complexes) best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse.
The coagulation pathway is a cascade of events that leads to hemostasis. The intricate pathway allows for rapid healing and prevention of spontaneous bleeding. Two paths, intrinsic and extrinsic, originate separately but converge at a specific point, leading to fibrin activation. The purpose is to stabilize the platelet plug with a fibrin mesh ultimately.
Glycolysis is the metabolic process in which glucose is broken down to produce energy. It occurs in the cytoplasm of cells and can happen in the presence or absence of oxygen, leading to the production of pyruvate, ATP, and NADH. Glycolysis is essential for cellular respiration and involves a series of enzymatic reactions that convert glucose into pyruvate, which can then enter further metabolic pathways.
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis.
Insulin (from Latin insula, 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (INS) gene. It is the main anabolic hormone of the body.
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA).
