9 Chapter Nine: Principles of Blood Products, Chemotherapy, and Total Parenteral Nutrition
Learning Objectives
- Describe the types, indications and administration of blood products, as well as the associated risks.
- Explain the principles of chemotherapy, including mechanisms of action, side effects and the nurse’s role in patient management during treatment.
- Identify the components, indications, and administration techniques for total parenteral nutrition, along with potential compilation.
- Apply safety protocols and monitoring practices for patients receiving blood products, chemotherapy, and TPN to minimize adverse effects.
- Discuss the ethical implications related to blood product administration, chemotherapy, and TPN in patient care.
Nursing in Louisiana
At St. Martinville Regional Hospital in southern Louisiana, the rhythm of healing beats steadily against the backdrop of bayou life. For Nurse Isabelle Dupre, each shift brings patients whose stories reflect the breadth and complexity of modern medicine.
That evening, she cared for Mr. LeBlanc, a local elder undergoing chemotherapy; Mrs. Broussard, a retired teacher rebuilding her strength with Total Parenteral Nutrition; and Mr. Alvarez, a young accident victim in need of a critical blood transfusion. Each treatment—whether chemotherapy, TPN, or transfusion—required precision, vigilance, and deep compassion.
In a town rich with cultural diversity, it’s not just advanced medical care that heals—it’s the human connection. For Isabelle, every patient encounter is a reminder that healing is as much about empathy as it is about expertise.
Introduction to Blood Products
Before administering blood products, the nurse must verify the provider’s order—including the product type, amount, rate, timing, and any special instructions—with another qualified provider (AABB, 2018; The Joint Commission, 2021). The nurse should assess relevant lab values, check for any required pre- or post-transfusion medications, and confirm the transfusion’s purpose. Patients should be informed about the procedure and asked about prior transfusion experiences, including any reactions or need for premedication. Premedication may be necessary for those with a history of allergic or febrile reactions, multiple transfusions, chronic illness, or autoimmune conditions. Once the blood bank confirms product availability, the nurse should confirm blood type and crossmatch, collect baseline vital signs, and notify the provider if the patient’s temperature exceeds 100°F (37.8°C).
Blood transfusions involve administering blood or its components to patients who have experienced significant blood loss or have conditions impairing blood cell production. The primary components include: Packed Red Blood Cells (PRBCs) are primarily used to treat anemia and blood loss by increasing the blood’s oxygen-carrying capacity. Platelets play a critical role in blood clotting and are transfused in patients with thrombocytopenia or active bleeding. Plasma, the liquid portion of blood, contains vital clotting factors and is often used in patients with clotting disorders, such as those caused by liver disease or anticoagulant overdose. Cryoprecipitate is a plasma-derived product rich in fibrinogen and other clotting factors, and it is typically administered to patients with bleeding disorders like disseminated intravascular coagulation (DIC) or low fibrinogen levels.
In cancer patients, blood transfusions are often necessary due to treatments like chemotherapy, which can suppress bone marrow function, leading to anemia and thrombocytopenia. Transfusions help manage these complications, improving patient outcomes (Cancer.org). Chemotherapy involves using drugs to kill or inhibit the growth of cancer cells. These agents target rapidly dividing cells, a hallmark of cancer. While effective, chemotherapy can also affect normal cells, leading to side effects such as nausea, fatigue, and immunosuppression. Advancements in targeted therapies and immunotherapies aim to improve efficacy and reduce adverse effects.
Cultural Considerations: Blood and Blood Products Considerations for Jehovah’s Witnesses
Jehovah’s Witnesses may decline the use of blood or blood product transfusions based on their religious beliefs. While many refuse whole blood and its primary components (red cells, white cells, platelets, and plasma), some may accept certain blood derivatives, such as albumin, clotting factors, and immunoglobulins. It is essential to assess each patient’s individual beliefs and not assume refusal of treatment. Provide education on the clinical indication for the transfusion, engage in open discussion to understand the patient’s preferences, and respect their informed decision. If a patient declines a transfusion, ensure the refusal is clearly documented and promptly communicated to the healthcare provider. (2025 Openstax)
Blood Typing and Crossmatching
Blood typing and crossmatching are critical steps in ensuring the safety and compatibility of blood transfusions. Blood typing identifies a person’s ABO blood group (A, B, AB, or O) and Rh factor (positive or negative). This is essential to prevent serious reactions caused by incompatibility between donor and recipient blood types.
Crossmatching is a more specific test done after blood typing. It involves mixing a sample of the patient’s blood with a sample of the donor’s blood to check for any reactions. If the mixture shows no signs of clumping (agglutination) or hemolysis, the blood is considered compatible.
These steps help prevent hemolytic transfusion reactions, which can occur if the recipient’s immune system attacks transfused red blood cells. Blood typing and crossmatching are mandatory before transfusions of red blood cells and are also recommended for certain plasma or platelet transfusions, especially in patients with a history of transfusion reactions or alloantibodies.
Blood Donor Compatibility
Ensuring donor-recipient blood compatibility is critical for the safety and effectiveness of blood transfusions. Incompatible transfusions can provoke immune responses due to the presence of antibodies targeting foreign blood antigens.
- Individuals with type A blood may receive type A or O blood.
- Individuals with type B blood may receive type B or O blood.
- Individuals with type AB blood are universal recipients and may receive type A, B, AB, or O blood.
- Individuals with type O blood are considered universal donors, as their blood can be transfused to recipients of any ABO blood type. However, they can only receive type O blood themselves.
Type O blood is particularly valuable in emergency situations where immediate transfusion is required and blood typing has not yet been completed.
In addition to ABO compatibility, the Rh factor is another critical component. Individuals with Rh-positive (Rh+) blood can receive both Rh+ and Rh− blood. However, those with Rh-negative (Rh− blood) should only receive Rh− blood to avoid potential sensitization or adverse reactions. For a transfusion to be considered safe, both ABO blood type and Rh factor compatibility must be confirmed.
Table 9.1
Blood Compatibility by Type and Rh Factor
| Blood Type | Can Donate To | Can Receive From |
|---|---|---|
| A+ | A+, AB+ | A+, A−, O+, O− |
| O+ | O+, A+, B+, AB+ | O+, O− |
| B+ | B+, AB+ | B+, B−, O+, O− |
| AB+ | AB+ | All types |
| A− | A+, A−, AB+, AB− | A−, O− |
| O− | All types | O− |
| B− | B+, B−, AB+, AB− | B−, O− |
| AB− | AB+, AB− | AB−, A−, B−, O− |
Blood Products

Blood products are components derived from whole blood that are used in medical treatments. These products are vital for managing patients with various medical conditions, including those with anemia, bleeding disorders, or trauma. Blood transfusions can be life-saving and are used to treat a variety of conditions, but they require careful monitoring to avoid complications.
Blood products are essential components used in medical treatment, each with specific functions and indications. Whole blood is the most basic form, containing red blood cells (RBCs), plasma, white blood cells, and platelets. However, it is rarely used today, except in cases of massive blood loss from trauma or surgery, as its components are often separated and administered individually. Red Blood Cells (RBCs) are critical for oxygen transport from the lungs to the body and returning carbon dioxide to the lungs for exhalation. RBCs are commonly used to treat patients with anemia, significant blood loss, or low hemoglobin levels, and are typically transfused over 2-4 hours via IV.
Platelets are essential for clot formation and preventing bleeding, particularly in patients with thrombocytopenia due to conditions like chemotherapy or bleeding disorders such as hemophilia. Platelet transfusions are administered more rapidly through IV infusion compared to RBCs. Fresh Frozen Plasma (FFP) is the liquid portion of blood containing proteins, electrolytes, and clotting factors. It is indicated for patients with clotting disorders, such as those caused by liver disease, massive transfusion, or warfarin toxicity, and is usually infused over 30-60 minutes via IV. Cryoprecipitate is a plasma component rich in clotting factors like fibrinogen, factor VIII, and von Willebrand factor, making it vital for treating bleeding disorders related to low fibrinogen levels, such as in disseminated intravascular coagulation (DIC) or massive hemorrhage. It is administered through IV infusion over approximately 30 minutes. Lastly, Albumin, a protein found in plasma, helps maintain oncotic pressure to prevent fluid from leaking into tissues. It is used for volume expansion in conditions such as hypovolemia, shock, or liver cirrhosis, and is generally administered via IV infusion over several hours, depending on the patient's condition.

Preparation for Administration of Blood and Blood Products
When administering blood or blood products, it is essential to use tubing specifically labeled for blood administration. This specialized blood tubing is Y-shaped and includes a micromesh filter designed to prevent the infusion of blood clots and particulate matter into the patient.
Before beginning the setup, ensure all roller clamps are closed—two located above the filter and one below it. Using one of the Y-ports, spike a bag of 0.9% normal saline. Open the roller clamp above the Y-site on the saline side, then gently squeeze the drip chamber to allow saline to saturate the filter and fill approximately one-third to one-half of the chamber.
Next, open the roller clamp below the drip chamber to prime the tubing, allowing saline to flow through to the distal end. Once the tubing is fully primed, close the lower roller clamp to maintain readiness for blood product administration. Carefully spike the blood bag using the Y-type IV tubing by inserting the spike into the designated port, taking care not to puncture any part of the blood bag outside the port area. Ensure the spike is fully inserted into the port. Open the roller clamp on the blood Y-tubing and gently squeeze the filter chamber until it is completely filled with blood. Label the blood tubing with the current date and time, as it must be replaced at least every four hours to minimize the risk of bacterial contamination.
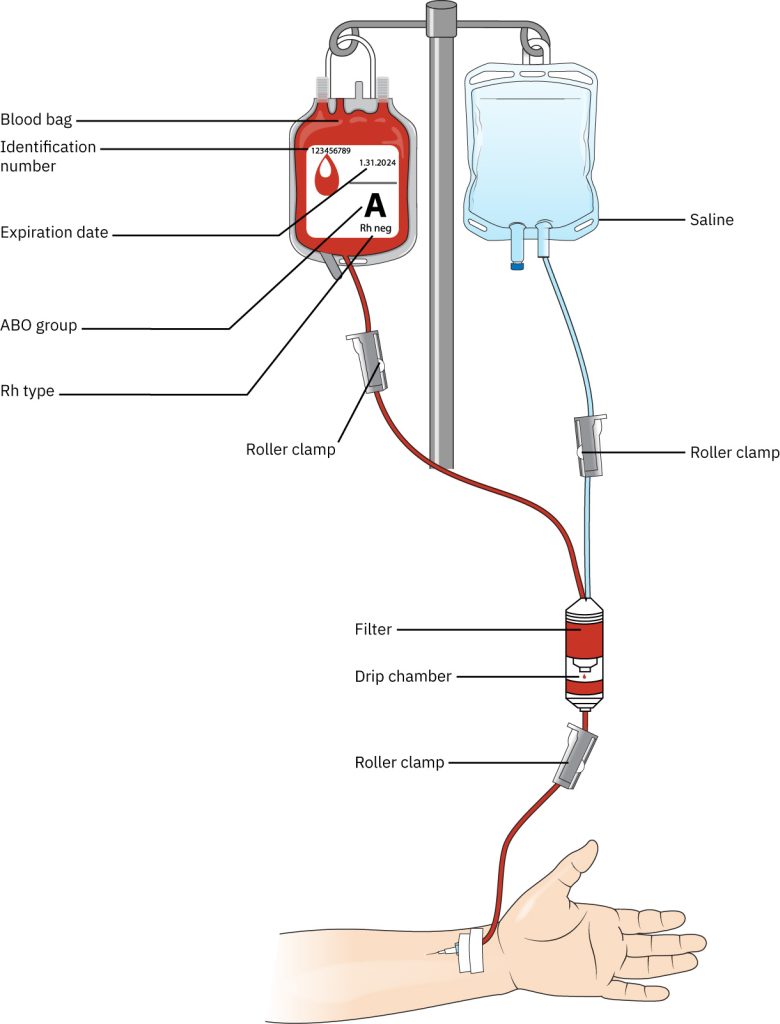
Indications for Blood Transfusion
Blood transfusions are indicated in various clinical situations, including:
- Anemia: A condition where there is a shortage of red blood cells, leading to fatigue and weakness. Blood products like RBCs are used to restore normal hemoglobin levels.
- Trauma: Patients with severe blood loss due to accidents or surgeries may require red blood cell transfusions to restore blood volume and oxygen-carrying capacity.
- Hemophilia or Bleeding Disorders: Platelets or clotting factors are used to control bleeding in patients with clotting disorders.
- Burns or Shock: Plasma or albumin can be administered to maintain fluid balance in critically ill patients.
- Surgical Procedures: Major surgeries may require blood transfusions to replace blood lost during the procedure.
Administration of Blood Products
Pre-Transfusion Verification Protocols
Upon receiving blood or blood products from the blood bank, two licensed nurses must conduct a thorough verification process. This includes confirming the provider’s order and ensuring that the information on the blood product label precisely matches the details on the patient’s blood bank identification bracelet, in accordance with AABB guidelines (2018). One nurse should read aloud from one source, while the second nurse cross-checks the corresponding information from the other source.
The verification must include confirmation of the serial number, blood component, blood type, Rh factor, and expiration date. Additionally, the patient's identity must be confirmed by comparing the blood product label with the patient’s identification bracelet, including the patient’s full name, date of birth, and medical record number.
Both nurses are required to document the verification process in alignment with institutional policies. If any inconsistencies or discrepancies are identified, the transfusion must not be initiated. Instead, the blood bank should be notified immediately. Blood products are typically administered through a large-bore IV catheter or a central venous line to ensure adequate flow and minimize the risk of complications (American Red Cross, 2023).
Initiating the Transfusion
Monitor Vital Signs
It is essential to document baseline vital signs, including blood pressure, pulse, temperature, and respiratory rate, prior to initiating the transfusion. Continuous monitoring of these vital signs is critical, especially within the first 15 minutes, to detect any early signs of a transfusion reaction (AABB, 2023).
Rate of Infusion
Healthcare providers should adhere to institutional guidelines regarding the recommended rate of transfusion for different blood products. For red blood cells (RBCs), the typical transfusion time is 2-4 hours, though this may vary depending on the patient’s condition and specific protocols (World Health Organization, 2022).
Observation
Close observation is required during the transfusion, particularly in the first 15 minutes, for signs of adverse reactions such as fever, chills, back pain, or rash. Immediate intervention may be necessary if any complications arise (National Blood Authority, 2023).
Post-Transfusion
Continue to monitor the patient’s vital signs for 30 minutes after the transfusion ends. Access for signs of reactions such as fever, rash, shortness of breath, or hematuria should be reported immediately. Record the details of the transfusion, including the product type, volume, start and end times, and any reactions or complications.
Potential Risks and Reactions
While blood transfusions are considered generally safe, healthcare providers must remain vigilant regarding the potential risks and adverse reactions that can arise during or after the procedure.
Allergic Reactions
These can range from mild symptoms like itching or a rash to more severe reactions, including anaphylaxis, although the latter is rare. Allergic reactions are often related to proteins in the donor blood (American Red Cross, 2023).
Fever and Chills
These are common reactions that can occur due to the presence of white blood cells in the transfused blood. This type of response is typically self-limiting but may require antipyretics or slowing the transfusion rate (AABB, 2023).
Hemolytic Reactions
These occur when there is a blood type mismatch between the donor and recipient, resulting in the destruction of red blood cells. Such reactions can be life-threatening and are why rigorous blood typing and crossmatching are crucial before transfusion (World Health Organization, 2022).
Infection
While blood products undergo extensive screening to detect and eliminate pathogens, there remains a very small risk of transmitting infections such as HIV, hepatitis B, or hepatitis C. Advances in testing technology have significantly reduced this risk, but it cannot be entirely eliminated (Centers for Disease Control and Prevention [CDC], 2023).
Volume Overload
Over-transfusion of blood products can lead to circulatory overload, which can result in complications such as pulmonary edema. This is particularly a concern in vulnerable populations, including patients with heart failure or kidney dysfunction (National Blood Authority, 2023).
Principles of Chemotherapy
Chemotherapy involves the use of chemical agents to treat cancer by targeting and destroying rapidly dividing cells. Understanding the mechanisms of action of these agents and adhering to safety protocols are essential for effective treatment and minimizing risks to patients and healthcare providers.
Mechanisms of Action
Chemotherapeutic agents are classified based on their mechanisms of action:
- Alkylating Agents: These drugs add alkyl groups to DNA, leading to DNA strand breaks and inhibiting cell division. Examples include nitrogen mustards (e.g., cyclophosphamide), nitrosoureas (e.g., carmustine), and platinum analogs (e.g., cisplatin) (ncbi.nlm.nih.gov)
- Antimetabolites: These agents mimic natural substances, interfering with DNA and RNA synthesis. Methotrexate, for instance, inhibits folic acid metabolism, essential for DNA synthesis
- Antitumor Antibiotics: These drugs bind to DNA, inhibiting transcription and replication. Doxorubicin is a well-known example.
- Mitotic Inhibitors: These agents disrupt microtubule function, preventing cell division. Vincristine and vinblastine are examples.
- Topoisomerase Inhibitors: These drugs interfere with enzymes that manage DNA supercoiling, essential for DNA replication. Etoposide and irinotecan are examples.
(Oncology Pro)
Safety Protocols
Handling chemotherapy drugs requires stringent safety measures to protect both patients and healthcare workers. Healthcare providers must wear appropriate personal protective equipment (PPE), including gloves, gowns, masks, and eye protection, to prevent exposure during administration (cancervic.org.au). Chemotherapy should only be given in designated areas with proper ventilation systems to minimize exposure, while chemotherapy drugs and their waste products must be disposed of according to institutional protocols to prevent environmental contamination. Additionally, patient education is vital—patients should be informed about potential side effects and the importance of promptly reporting any adverse reactions (cancervic.org.au).
Adhering to these safety protocols is crucial to minimize risks associated with chemotherapy administration
9.1
Items to Avoid While Receiving Chemotherapy
| Item to Avoid | Rationale |
|---|---|
| Seafood and Raw Meat | Chemotherapy weakens the immune system, making it easier for bacteria, viruses, and parasites to cause infections. Seafood and raw meat, especially if not properly cooked, may contain harmful bacteria like Salmonella or E. coli, which can increase the risk of foodborne infections. |
| Spicy and Acidic Foods | Chemotherapy can cause mouth sores, nausea, or gastrointestinal discomfort. Spicy and acidic foods may exacerbate these symptoms, causing irritation to the mouth, throat, or stomach lining, making it more difficult to eat and maintain proper nutrition. |
| New Medications or Supplements | New medications or supplements may interact with chemotherapy drugs, potentially reducing their effectiveness or causing harmful side effects. It's important to consult with a healthcare provider before introducing any new substances to ensure safety and avoid complications. |
| Avoid Alcohol and Smoking | Alcohol can irritate the stomach and liver, which are already stressed during chemotherapy, and it may also interfere with the body’s ability to heal. Smoking increases the risk of infections and delays recovery by further weakening the immune system, in addition to increasing the risk of cancer recurrence. |
| Avoid Sun Exposure | Chemotherapy can make the skin more sensitive to sunlight, increasing the risk of sunburns and skin damage. Certain chemotherapy drugs may also make the skin more prone to rashes or reactions from UV exposure. Protecting the skin is crucial to prevent burns or other complications. |
| Pregnancy and Exposure of Sexual Partner to Chemo | Chemotherapy drugs can be harmful to a developing fetus, potentially causing birth defects or miscarriage. It's recommended to avoid pregnancy during chemotherapy, and both the patient and their sexual partner should take precautions, such as using contraception, to prevent exposure to chemotherapy drugs during sexual activity. |
| Caregiver should take precautions when doing laundry and cleaning | Chemotherapy drugs can be excreted in bodily fluids such as urine, sweat, and saliva. Caregivers should handle clothing, bedding, and personal items with care, wearing gloves if necessary, to avoid direct contact with these fluids and reduce the risk of exposure to toxic substances. |
| Avoid sick people | Chemotherapy suppresses the immune system, leaving patients more vulnerable to infections. Avoiding contact with sick people helps reduce the risk of contracting illnesses such as the flu, colds, or other viral and bacterial infections, which can be more severe or harder to treat while undergoing chemotherapy. |
| Expend energy | Chemotherapy can cause fatigue and decreased energy levels. It's essential to conserve energy by resting and prioritizing activities. Pushing through excessive activity may lead to increased exhaustion, making it harder for the body to recover and maintain its strength during treatment. |
| Withhold concerns and questions | It's important for patients to openly communicate with their healthcare team to understand their treatment plan, potential side effects, and precautions. Expressing concerns allows for timely adjustments to medications or lifestyle recommendations, ensuring that patients feel more informed and empowered during their chemotherapy journey. |
include mdanderson source
Total Parenteral Nutrition
Total Parenteral Nutrition (TPN) is a method of delivering complete nutrition intravenously to patients unable to use their digestive systems. It provides essential nutrients—proteins, carbohydrates, fats, vitamins, and minerals—bypassing the gastrointestinal tract. Indications for TPN include:
- Non Functional Gastrointestinal Tract: Conditions like short bowel syndrome or severe pancreatitis.
- Inability to Absorb Nutrients: Due to diseases like Crohn's or ulcerative colitis.
In cancer patients, TPN may be necessary when treatments impair the digestive system, ensuring adequate nutrition and supporting recovery (ncbi.nlm.nih.gov)
Understanding these components is crucial for healthcare professionals in providing comprehensive care to patients, particularly those with complex conditions requiring multidisciplinary management.
Indications for TPN
TPN is indicated in various clinical scenarios, including:
- Gastrointestinal Disorders: Conditions such as Crohn's disease, short bowel syndrome, or severe pancreatitis may necessitate TPN due to compromised digestive function.
- Cancer Treatments: Patients undergoing chemotherapy or radiation therapy may experience gastrointestinal side effects that impair nutrient absorption, making TPN essential.
- Critical Illness: Severe burns, trauma, or sepsis can lead to increased metabolic demands and gastrointestinal dysfunction, requiring TPN support.
Components of TPN
A typical TPN solution includes:
- Macronutrients:
- Proteins: Amino acids are provided to support tissue repair and immune function.
- Carbohydrates: Dextrose serves as the primary energy source.
- Fats: Lipids supply essential fatty acids and additional calories.
- Micronutrients:
- Vitamins and Minerals: These are included to prevent deficiencies and support metabolic processes.
- Electrolytes: Sodium, potassium, calcium, magnesium, and phosphate are adjusted based on individual patient needs.
- Administration and Monitoring TPN

TPN is typically administered through a central venous catheter to ensure adequate flow and reduce the risk of complications. Regular monitoring is essential to assess:
- Nutritional Status: Evaluating weight, serum albumin, and prealbumin levels.
- Electrolyte Balance: Regular blood tests to monitor levels of sodium, potassium, calcium, and phosphate.
- Liver Function: Monitoring liver enzymes to detect potential hepatobiliary complications.
- Infection Control: Observing for signs of catheter-related infections.
Safety Components
Adhering to safety protocols is essential to minimize the risks associated with total parenteral nutrition (TPN). This includes maintaining sterile technique during preparation and administration to prevent infections, ensuring compatibility checks to confirm that all TPN components are compatible and free from precipitates or emboli, and gradually initiating and weaning the treatment to avoid metabolic disturbances. Additionally, patient education plays a crucial role, as informing patients and caregivers about the importance of hygiene, recognizing potential complications, and adhering to prescribed protocols helps ensure the safe and effective use of TPN. For comprehensive guidelines and recommendations on TPN, refer to the American Society for Parenteral and Enteral Nutrition (ASPEN) resources. By adhering to these guidelines and protocols, healthcare providers can optimize the benefits of TPN while minimizing potential risks.
Patient Safety and Monitoring
Ensuring patient safety and effective monitoring during Total Parenteral Nutrition (TPN) is essential to prevent complications and optimize nutritional support. Adhering to established guidelines and protocols is crucial for healthcare providers.
Monitoring Guidelines
The American Society for Parenteral and Enteral Nutrition (ASPEN) provides comprehensive guidelines for TPN administration and monitoring. These guidelines emphasize the importance of individualized care plans, regular assessment of nutritional status, and vigilant monitoring for potential complications. By adhering to these guidelines and protocols, healthcare providers can optimize the benefits of TPN while minimizing potential risks. Interdisciplinary collaboration is essential in the management of patients undergoing Total Parenteral Nutrition (TPN), blood transfusions, and chemotherapy. A coordinated approach among healthcare professionals—including physicians, nurses, dietitians, pharmacists, and laboratory technicians—ensures comprehensive care, enhances patient outcomes, and minimizes potential complications.
Total Parenteral Nutrition (TPN) (this is meant to be here? Or is it meant as a review/summary? Not sure I have the heading structure right for this and the two below)
- Nutritional Assessment and Planning: Dietitians assess the patient's nutritional needs, considering factors like metabolic demands, organ function, and concurrent treatments. They collaborate with physicians to tailor TPN formulations, ensuring appropriate caloric intake, macronutrient balance, and micronutrient supplementation.
- Pharmacy Involvement: Pharmacists prepare and verify TPN solutions, ensuring compatibility of nutrients and medications. They monitor for potential drug-nutrient interactions and adjust formulations based on the patient's response and laboratory results.
- Nursing Care: Nurses administer TPN, monitor for adverse reactions, and educate patients and caregivers on infusion procedures and potential complications. They play a crucial role in detecting and managing issues like catheter-related infections or metabolic disturbances.
Chemotherapy
- Treatment Planning: Oncologists design chemotherapy regimens based on cancer type, stage, and patient health. They work with multidisciplinary teams to address supportive care needs, including antiemetic therapy and pain management.
- Administration and Monitoring: Nurses administer chemotherapy agents, adhering to safety protocols to prevent extravasation and other complications. They monitor vital signs and laboratory values, collaborating with pharmacists to adjust medications as needed.
- Psychosocial Support: Social workers and counselors provide emotional support, address financial concerns, and connect patients with support groups, enhancing quality of life during treatment.
Benefits of Interdisciplinary Collaboration
- Comprehensive Care: Integrating diverse expertise ensures all aspects of patient health are addressed, from nutritional needs to emotional well-being.
- Improved Patient Outcomes: Coordinated efforts reduce the risk of complications, enhance treatment efficacy, and promote recovery.
- Efficient Resource Utilization: Collaboration minimizes redundant tests and procedures, optimizing healthcare resources and reducing costs.
Ethical and Legal Considerations
Healthcare providers must confront and resolve critical ethical and legal challenges when managing blood products, chemotherapy, and total parenteral nutrition (TPN) to ensure patient safety, uphold autonomy, and comply with regulations. Obtaining informed consent, honoring religious beliefs, ensuring equitable resource allocation, and mitigating the risk of disease transmission is imperative. Additionally, compliance with Federal drug administration (FDA) and American Association of Blood Banks (AABB) regulations is non-negotiable. Protecting patient confidentiality and managing liability for errors is essential. In chemotherapy, providers face significant ethical dilemmas surrounding quality of life, patient autonomy, and the use of experimental treatments, particularly in terminal cases. Legally, adherence to FDA drug approvals is mandatory, and it is crucial to prevent medical malpractice and adeptly handle insurance disputes. For TPN, critical ethical issues include ensuring informed consent, making sound end-of-life decisions, and judicious resource allocation, as TPN may not always be beneficial. Legal obligations require strict adherence to USP standards for sterility, proactive management of malpractice risks, resolution of insurance coverage disputes, and recognition of a patient's right to refuse nutrition.
In conclusion, healthcare providers must assertively balance ethical principles with legal requirements to protect patients' rights and ensure the delivery of safe, effective, and responsible treatment.
Chapter Closer
A 75-year-old male patient with colon cancer has been receiving chemotherapy for the past few months. He is now experiencing significant fatigue, nausea, and a low white blood cell count. How would you manage these symptoms, and what should be monitored to ensure his safety during treatment?
Answer: To manage fatigue and nausea, consider administering anti-nausea medications, adjusting chemotherapy doses (if needed), and ensuring the patient gets adequate rest. For the low white blood cell count (neutropenia), consider administering growth factors such as filgrastim (G-CSF) to stimulate white blood cell production. Close monitoring of vital signs, white blood cell count, and signs of infection is essential. The patient’s hydration and nutrition should also be supported to improve overall well-being during chemotherapy.
Key Takeaways
- Some cohort members prepared this as a bulleted list
Review Questions
