Defining Protein
Protein makes up approximately 20 percent of the human body and is present in every single cell. The word protein is a Greek word, meaning “of utmost importance.” Proteins are called the workhorses of life as they provide the body with structure and perform various functions. You can stand, walk, run, skate, swim, and more because of your protein-rich muscles. Protein is necessary for proper immune system function, digestion, and hair and nail growth, and is involved in numerous other body functions. In fact, it is estimated that more than one hundred thousand different proteins exist within the human body. Proteins are crucial for the nourishment, renewal, and continuance of life. In this chapter, you will learn about the components of protein, the important roles that protein serves within the body, how the body uses protein, the risks and consequences associated with too much or too little protein, and where to find healthy sources of it in your diet.
What Is Protein?
Proteins, simply put, are macromolecules composed of amino acids. Amino acids are commonly called protein’s building blocks. Amino acids contain the elements carbon, hydrogen, and oxygen just as carbohydrates and lipids do, but proteins are the only macronutrient that contains nitrogen. In each amino acid the elements are arranged in a specific conformation around a central carbon atom The central carbon is connected to a side chain, a hydrogen, a nitrogen-containing amino group, and a carboxylic acid group—hence the name “amino acid.” There are Amino acids differ from each other by which specific side chain is bonded to the central carbon.
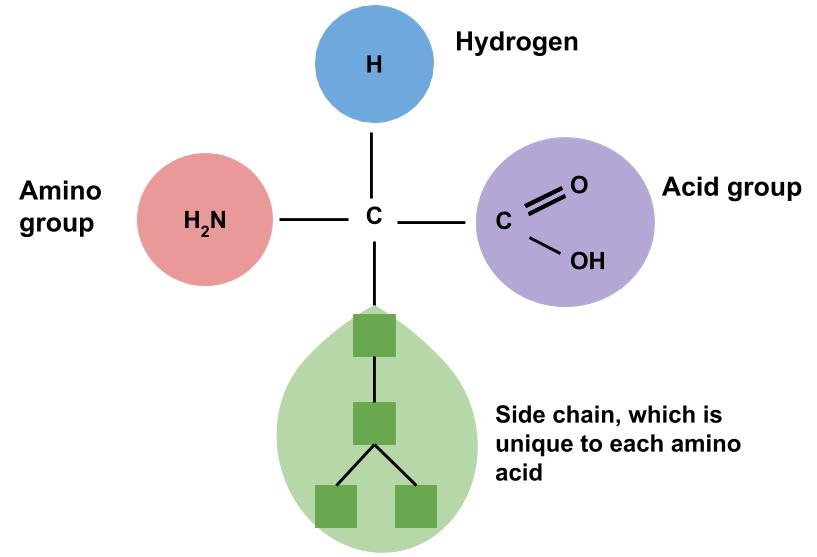
(Source: University of Hawaii @ Manoa, Allison Calabrese, CC-BY-NC-SA)
The arrangement of elements around the carbon center is the same for all amino acids. Only the side chain (R) differs.
It’s All in the Side Chain
The side chain of an amino acid, sometimes called the “R” group, can be as simple as one hydrogen or as complex as a six-carbon ring. Amino acids are classified according to the chemical characteristics of the side chain.
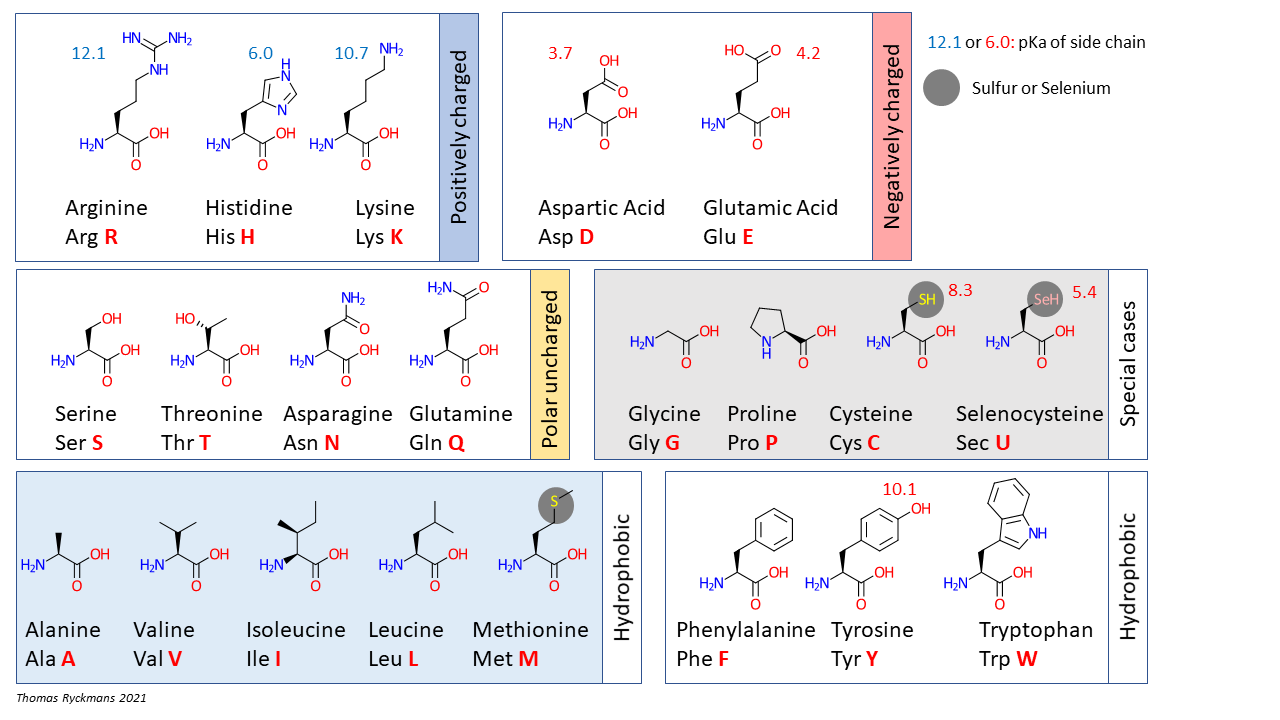
(Source: Wikimedia Commons, Thomas.ryckmans68, CC-BY-SA)
Essential and Nonessential Amino Acids
There are twenty amino acids. Eleven of these are called nonessential amino acids because the body can synthesize them. However, nine of the amino acids are called essential amino acids because we cannot synthesize them either at all or in sufficient amounts so they. These must be obtained from the diet. Sometimes during infancy, growth, and in diseased states the body cannot synthesize enough of some of the nonessential amino acids and more of them are required in the diet. These types of amino acids are called conditionally essential amino acids. The nutritional value of a protein is dependent on what amino acids it contains and in what quantities.
| Essential | Nonessential |
|---|---|
| Histidine | Alanine |
| Isoleucine | Arginine* |
| Leucine | Asparagine |
| Lysine | Aspartic acid |
| Methionine | Cysteine* |
| Phenylalanine | Glutamic acid |
| Threonine | Glutamine* |
| Tryptophan | Glycine* |
| Valine | Proline* |
| Serine | |
| Tyrosine* | |
| *Conditionally essential |
The Many Different Types of Proteins
Proteins come in many different shapes and sizes. The hormone insulin, which regulates blood glucose, is globular in shape and composed of only fifty-one amino acids. In contrast, collagen consists of more than one thousand amino acids and is shaped like a steel cable to provide tensile strength to tendons, ligaments, and bone. Titin, which gives elasticity to skeletal muscle, is curvy-shaped and is the largest known protein with more than twenty-five thousand amino acids! The abundant variations of proteins are due to the unending number of amino acid sequences that can be formed. Consider music to compare how so many different proteins can be designed from only twenty amino acids. All of the music that exists in the world has been derived from a basic set of 12 notes C, C#/D♭, D, D#/E♭, E, F, F#/G♭, G, G#/A♭, A, A#/B♭, and B. As a result, there is a vast array of music and songs all composed of specific sequences from these basic musical notes. Similarly, the twenty amino acids can be linked in an extraordinary number of sequences, much more than possible for the seven musical notes to create songs. As a result, there are enormous variations in amino acid sequences that can be made. For example, if an amino acid sequence for a protein is 104 amino acids long, the possible combinations of amino acid sequences are equal to 20104, which is two followed by 135 zeros!
Building Proteins with Amino Acids
Dehydration synthesis forms peptide bonds between amino acids. (Source: Wikimedia Commons, YassineMrabet, Public Domain)
Each amino acid is connected to the next amino acid by a special chemical bond called a peptide bond. The peptide bond forms between the carboxylic acid group of one amino acid and the amino group of another, releasing a molecule of water.
Protein Organization
Protein’s structure enables it to perform a variety of functions. Proteins are similar to carbohydrates and lipids in that they are polymers of simple repeating units; however, proteins are much more structurally complex. In contrast to carbohydrates, which have identical repeating units, proteins are made up of different amino acids. Furthermore, a protein is organized into four different structural levels.
Primary: The first level is the one-dimensional sequence of amino acids that are held together by peptide bonds. Carbohydrates and lipids are also one-dimensional sequences of their respective monomers, which may be branched, coiled, fibrous, or globular. Still, their conformation is much more random and is not organized by their sequence of monomers.
Secondary: The second protein structure level depends on the chemical interactions between amino acids, which cause the protein to fold into a specific shape, such as a helix (like a coiled spring) or sheet.
Tertiary: The third level of protein structure is three-dimensional. As the different side chains of amino acids chemically interact, they either repel or attract each other, resulting in the folded structure. Thus, the specific sequence of amino acids in a protein directs the protein to fold into a specific, organized shape.
Quaternary: The fourth level of structure is achieved when protein fragments called peptides combine to make one larger functional protein. The protein hemoglobin is an example of a protein that has quaternary structure. It comprises four peptides that bond together to form a functional oxygen carrier.
A protein’s structure also influences its nutritional quality. Large fibrous protein structures are more challenging to digest than smaller proteins, and some, such as keratin, are indigestible. Because digestion of some fibrous proteins is incomplete, not all of the amino acids are absorbed and available for the body to utilize, thereby decreasing their nutritional value.
Each protein in the human body differs in its amino acid sequence and consequently, its shape. The newly synthesized protein is structured to perform a particular function in a cell. A protein made with an incorrectly placed amino acid may not function properly and this can sometimes cause disease.
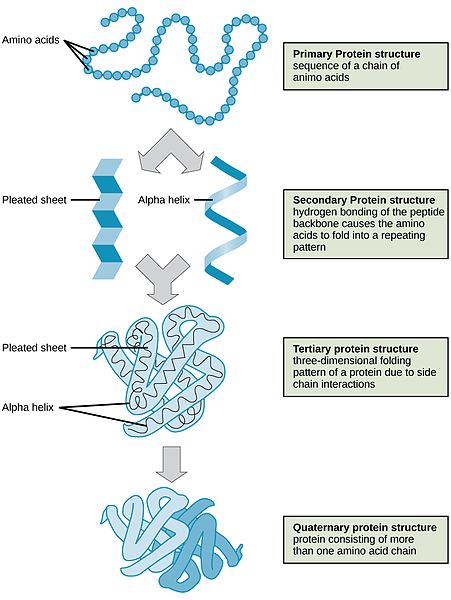
(Source Wikimedia Commons, OpenStax, CC-BY)
Learning Activities
Technology Note: The second edition of the Human Nutrition Open Educational Resource (OER) textbook features interactive learning activities. These activities are available in the web-based textbook and are not in downloadable versions (EPUB, Digital PDF, Print_PDF, or Open Document).
Learning activities may be used across various mobile devices; however, for the best user experience, it is strongly recommended that users complete these activities using a desktop or laptop computer.
A class of compounds composed of linked amino acids. They contain carbon, hydrogen, nitrogen, oxygen, and sometimes other atoms in specific configurations.
The molecules from which proteins are built, each protein being composed of a specific sequence of linked amino acids.
Essential nutrients that are needed by the body in large amounts
The smallest unit of an element.
An amino acid that can be synthesized by the human body in sufficient quantities to meet the body’s needs.
An amino acid that cannot be synthesized by the body or synthesized in sufficient quantities to meet the body’s needs.
A hormone secreted by the pancreas in response to elevated blood glucose levels to transport glucose into the muscle or fat cells.
The chemical linkage between amino acids into the structure of a protein chain.
Two or more amino acids joined together by peptide bonds.
An iron-containing protein found in red blood cells that bind and transports oxygen throughout the body.

