49 Community Ecology Lab
Lab adapted by Staci Forgey and Dr. James Holden, Tidewater Community College biology faculty, with permission from Dr. William Edwards, biology faculty at Niagara University.
Lab Objectives
- Describe the processes of primary succession and secondary succession
- Explain what makes a community and an ecosystem different
- Describe the differences between abiotic and biotic factors
- Explain why disturbances play an important role in the progression of succession
- Define a climax community
- Describe why most areas will not make it to a climax community
- Describe the plant communities present after glacial succession and how they change the environment
- Explain the stages of succession of milk
- Describe how pH changes as milk goes through successional stages
- Explain the difference between Gram-negative and positive bacteria
- Draw and describe the shapes of bacteria
- Formulate a hypothesis based on background data
http://www.slideshare.net/CandelaContent/succession-51120026
Download a PDF of the lab to print:
Ecological Succession of Bacteria in Milk
The communities within ecosystems develop over time, from very simple species assemblages, to complex, rich ecosystems. In this process, called succession, each succeeding species facilitates changes in the environment which allow new species to come into the ecosystem. As the community becomes more and more complex, the biodiversity of the ecosystem also increases. Both biotic and abiotic processes can reset the succession process. That is, events caused by both the community itself and outside events can return the community to an earlier succession state. The gradual changes in the community are both orderly and predictable in many ecosystems. The peak or most complex, advanced community that can develop in any abiotic environment is called the climax community. The picture below describes the developing communities as a series of steps, each of which can be driven against the succession process by disturbances:
Question
- What types of events could “reset” a succession process? Name at least one biotic and one abiotic disturbance.
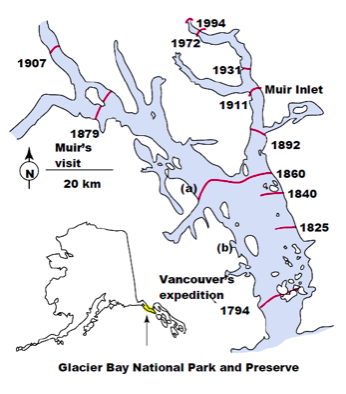
One example of a severe disturbance, reducing the land to bare ground, is the passage of a glacier. Though glaciers have not covered this part of North America for ten thousand years, there are parts of the continent that are even now being uncovered by receding glaciers. One area is the Pacific Northwest. From Juneau to Glacier Bay, many glaciers that have previously fallen directly into the ocean are now leaving bare soil that has not been exposed for more than fifty thousand years. Because the glaciers retreat very slowly, we can watch communities change over time in a single snapshot. Take the glaciers of Glacier Bay National Park, midway between Juneau and Anchorage, Alaska. The glaciers there have been retreating since the explorer Vancouver’s first expedition in 1794. Since then, the retreat has covered over 100 km, including new coastlines, meadows, and mountains:
Question
- Explain how this retreat will result in different communities along the glacier’s retreat, though in similar environments. Hint: what is the difference between exposed soil at point A and point B?
As the glacier retreats, it leaves nutrient-poor soil that can only support simple plants such as liverworts, lichens, and other primitive plants. As they photosynthesize and die, we see them enter the decomposer pathway and increase the quality of the soil for later plants. However, this slow glacier retreat is a unique situation. To set up an experiment to test our understanding of succession would require hundreds of years, longer than a scientist’s lifetime. However, some organisms and communities proceed at a much faster rate, within your own refrigerators. The process of milk decomposition from a community of bacteria can test the same processes and theories in a much more reasonable time frame. This substitution of a simpler and faster community for experimental purposes is called a ‘model’ system.
Milk is a highly nutritious food containing carbohydrates (lactose, or milk sugar), proteins (casein, or curd), and lipids (butterfat). This high level of nutrition makes milk an excellent medium for the growth of bacteria. Pasteurizing milk does not sterilize it (sterilizing kills all bacteria) but merely destroys pathogenic bacteria, leaving many bacteria that can multiply and these bacteria will begin to grow and bring about milk spoilage. Biologists have discovered that as milk ages, changing conditions in the milk bring about a predictable, orderly succession of microorganism communities.
In this laboratory exercise, you will observe successional patterns in several types of milk. You will record changes in the environmental conditions of the types of milk as they age. These changes are a result of changes in the bacterial communities. Here are some of the major bacteria found at various stages.
Stages of Milk Succession
- Pseudomonas and Achromobacter (Gram-negative rods) digest butterfat and give milk a putrid smell
- Lactobacillus (Gram-positive rod) and Streptococcus (Gram-positive coccus) ferment lactose to lactic acid and acetic acid.
- Acidity sours milk and converts casein to curd.
- Yeast (fungi) thrive in acidic conditions and metabolize the acids into non-acidic compounds.
- Bacillus metabolize proteins into ammonia products and raise the milk’s pH. Spoiled milk odor is very noticeable at this stage.
Questions
- What are some advantages to using bacteria as opposed to plants in this experiment?
- What factors might speed or delay a successional process? Apply your example to succession in milk.
Locate the milk samples available in the lab. Take a look at the samples and form two conditions you would like to study. Develop a short design for studying the first condition, state the dependent and independent variable, control and experimental group, the hypothesis, and any variables that have been controlled. Then develop a short design for studying the second condition, state the dependent and independent variable, control and experimental group, the hypothesis, and any variables that have been controlled.
Just as in any of our experiments, you must use the scientific method effectively. Develop hypotheses (at least 2—one with different milk types and one with either temperature or time as an independent variable) that you can test in the process of the milk community succession.
We will be performing a Gram stain on our milk samples. Remember from our microbiology section that bacteria can be either Gram negative (pink) or Gram positive (purple). We will also look at these bacteria under a microscope to identify their shapes. Recall that bacteria can be cocci, bacilli, or spirillum.
Questions
- What hypotheses are you testing? List both here.
- What information led you to ask these hypotheses?
- Make predictions about your hypotheses. (i.e., How will you know if the data supports or refutes your hypotheses?)
- Be sure to identify the variables you will test, and those you will control for each experiment.
- Prepare a table for data collection. You will be recording the pH, smell, consistency, and bacteria shapes and colors present in your milk samples.
On each lab bench are several small beakers. You will obtain a sample of the milk samples you need for your experimental design and test the pH, color, consistency, smell, and other characteristics of each sample. For each milk sample:
- Using the Vernier, take the pH of each flask. Record your results.
- Record the color, odor (sour, putrid), and consistency (coagulation: slight, moderate, chunky) of the milk in each flask.
- Perform Gram staining as outlined below. Note: We will be using chemicals and open flames. Exercise caution when performing this portion of the lab.
Materials
- Microscope slide
- Bunsen burner and tubing
- Crystal violet (primary stain)
- Iodine solution / Gram’s Iodine (mordant that fixes crystal violet to cell wall)
- Decolorizer (e.g., ethanol)
- Safranin (secondary stain)
- Water (preferably in a squirt bottle)
Procedure
- Make a slide with your milk sample to be stained. Heat fix the sample to the slide by carefully passing the slide with a drop or small piece of sample on it through a Bunsen burner three times.
- Add the primary stain (crystal violet) to the sample/slide and let sit for 15 seconds. Rinse slide with a gentle stream of water for a maximum of 5 seconds to remove unbound crystal violet.
- Add Gram’s iodine for 15 seconds—this is an agent that fixes the crystal violet to the bacterial cell wall.
- Rinse sample/slide with acetone or alcohol for ~3 seconds and rinse with a gentle stream of water. The alcohol will decolorize the sample if it is Gram negative, removing the crystal violet. However, if the alcohol remains on the sample for too long, it may also decolorize Gram-positive cells.
- Add the secondary stain, safranin, to the slide and incubate for 15 seconds. Wash with a gentle stream of water for a maximum of 5 seconds. If the bacteria is Gram positive, it will retain the primary stain (crystal violet) and not take the secondary stain (safranin), causing it to look violet/purple under a microscope. If the bacteria is Gram negative, it will lose the primary stain and take the secondary stain, causing it to appear red when viewed under a microscope.
Questions
- Describe the changing sequence of organisms and corresponding environmental changes during succession in the milk samples. Which bacteria are in each of your milk samples?
- Describe the changing sequence of organisms and corresponding environmental changes during succession in chocolate milk. Do the results of your investigation match your hypothesis?
- Compare succession in one or more types of milk. Propose reasons for differences.
- Propose another experiment to test the environmental factors and/or organisms changing in your proposed scenario for milk succession.
- How could you improve your test of the hypotheses? Be specific!
- Identify what happened to the pH of the milk as time passed.
- Infer what the change in pH means about the populations of microorganisms in the milk.
Licensing & Attributions
CC licensed content, Original
Community Ecology Lab. Authored by: Dr. William Edwards. Provided by: Niagara University. Located at: https://www.niagara.edu/. License: CC BY: Attribution
CC LICENSED CONTENT, SHARED PREVIOUSLY
Biology 102 Labs. Authored by: Lynette Hauser & Dr. James Holden. Provided by: Tidewater Community College. Located at: https://www.tcc.edu/. License: Public Domain: No Known Copyright