Ionization Constants of Weak Acids
Paul Flowers; Edward J. Neth; William R. Robinson; Klaus Theopold; and Richard Langley
[latexpage]
| Ionization Constants of Weak Acids | |||
|---|---|---|---|
| Acid | Formula | Ka at 25 °C | Lewis Structure |
| acetic | CH3CO2H | 1.8 \(×\) 10−5 | 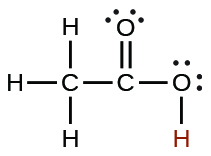 |
| arsenic | H3AsO4 | 5.5 \(×\) 10−3 | 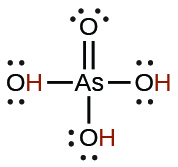 |
 |
1.7 \(×\) 10−7 | ||
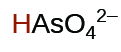 |
3.0 \(×\) 10−12 | ||
| arsenous | H3AsO3 | 5.1 \(×\) 10−10 | 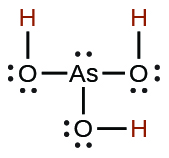 |
| boric | H3BO3 | 5.4 \(×\) 10−10 | 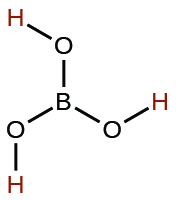 |
| carbonic | H2CO3 | 4.3 \(×\) 10−7 | 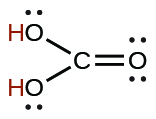 |
 |
4.7 \(×\) 10−11 | ||
| cyanic | HCNO | 2 \(×\) 10−4 | 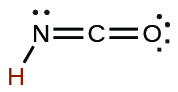 |
| formic | HCO2H | 1.8 \(×\) 10−4 | 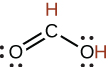 |
| hydrazoic | HN3 | 2.5 \(×\) 10−5 |  |
| hydrocyanic | HCN | 4.9 \(×\) 10−10 | |
| hydrofluoric | HF | 6.4 \(×\) 10−4 | |
| hydrogen peroxide | H2O2 | 2.4 \(×\) 10−12 | 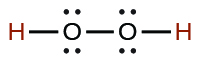 |
| hydrogen selenide | H2Se | 1.29 \(×\) 10−4 | |
| HSe– | 1 \(×\) 10−12 | ||
| hydrogen sulfate ion |  |
1.2 \(×\) 10−2 | 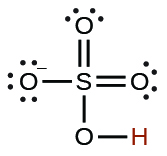 |
| hydrogen sulfide | H2S | 8.9 \(×\) 10−8 | |
| HS– | 1.0 \(×\) 10−19 | ||
| hydrogen telluride | H2Te | 2.3 \(×\) 10−3 | |
| HTe– | 1.6 \(×\) 10−11 | ||
| hypobromous | HBrO | 2.8 \(×\) 10−9 | |
| hypochlorous | HClO | 2.9 \(×\) 10−8 | |
| nitrous | HNO2 | 4.6 \(×\) 10−4 | 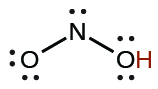 |
| oxalic | H2C2O4 | 6.0 \(×\) 10−2 | 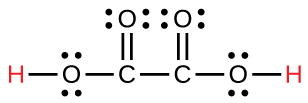 |
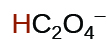 |
6.1 \(×\) 10−5 | ||
| phosphoric | H3PO4 | 7.5 \(×\) 10−3 | 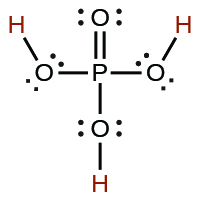 |
 |
6.2 \(×\) 10−8 | ||
 |
4.2 \(×\) 10−13 | ||
| phosphorous | H3PO3 | 5 \(×\) 10−2 | 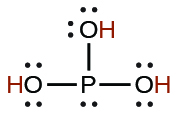 |
 |
2.0 \(×\) 10−7 | ||
| sulfurous | H2SO3 | 1.6 \(×\) 10−2 | 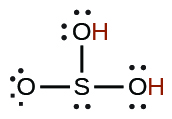 |
 |
6.4 \(×\) 10−8 | ||

