190 Batteries and Fuel Cells
Paul Flowers; Edward J. Neth; William R. Robinson; Klaus Theopold; and Richard Langley
[latexpage]
Learning Objectives
By the end of this section, you will be able to:
- Describe the electrochemistry associated with several common batteries
- Distinguish the operation of a fuel cell from that of a battery
There are many technological products associated with the past two centuries of electrochemistry research, none more immediately obvious than the battery. A battery is a galvanic cell that has been specially designed and constructed in a way that best suits its intended use a source of electrical power for specific applications. Among the first successful batteries was the Daniell cell, which relied on the spontaneous oxidation of zinc by copper(II) ions ((Figure)):
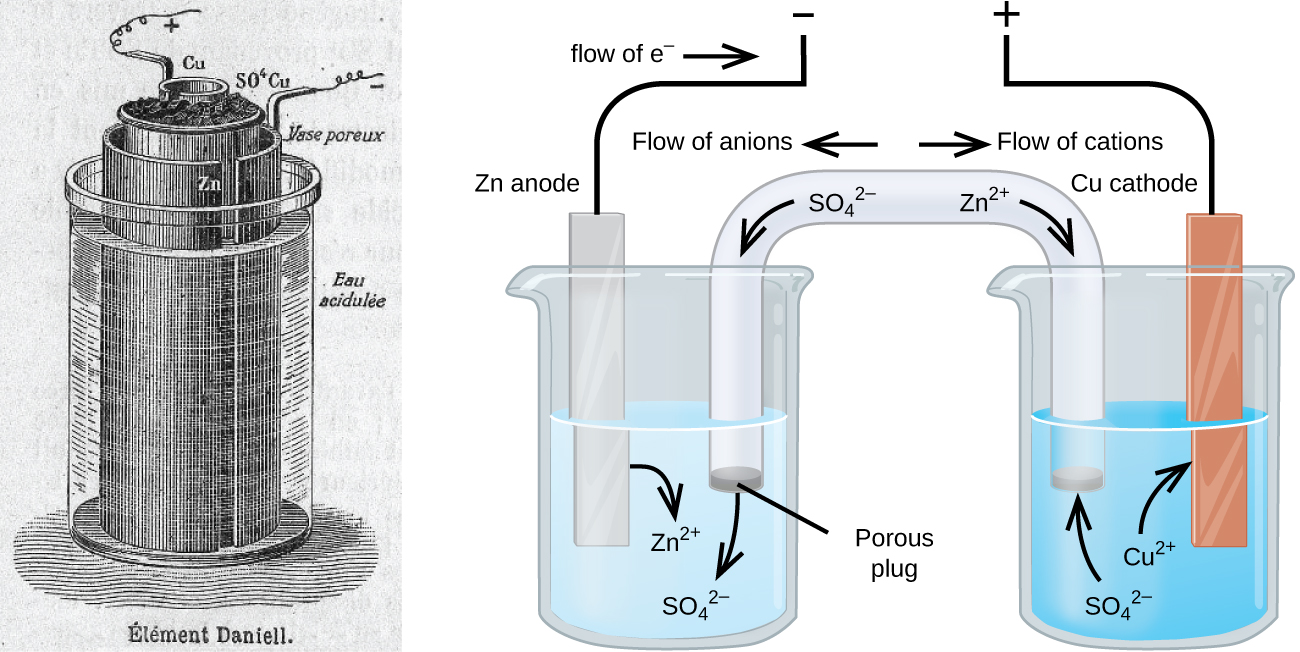
Modern batteries exist in a multitude of forms to accommodate various applications, from tiny button batteries that provide the modest power needs of a wristwatch to the very large batteries used to supply backup energy to municipal power grids. Some batteries are designed for single-use applications and cannot be recharged (primary cells), while others are based on conveniently reversible cell reactions that allow recharging by an external power source (secondary cells). This section will provide a summary of the basic electrochemical aspects of several batteries familiar to most consumers, and will introduce a related electrochemical device called a fuel cell that can offer improved performance in certain applications.
Visit this site to learn more about batteries.
Single-Use Batteries
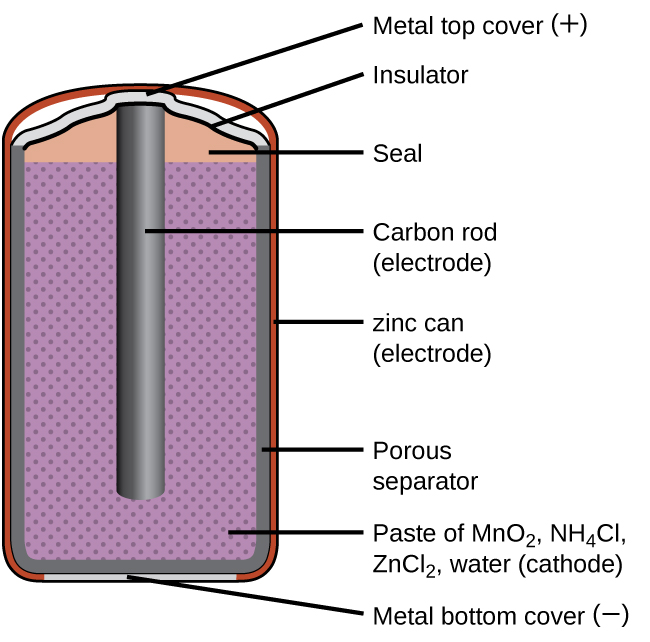
A common primary battery is the dry cell, which uses a zinc can as both container and anode (“–” terminal) and a graphite rod as the cathode (“+” terminal). The Zn can is filled with an electrolyte paste containing manganese(IV) oxide, zinc(II) chloride, ammonium chloride, and water. A graphite rod is immersed in the electrolyte paste to complete the cell. The spontaneous cell reaction involves the oxidation of zinc:
and the reduction of manganese(IV)
which together yield the cell reaction:
The voltage (cell potential) of a dry cell is approximately 1.5 V. Dry cells are available in various sizes (e.g., D, C, AA, AAA). All sizes of dry cells comprise the same components, and so they exhibit the same voltage, but larger cells contain greater amounts of the redox reactants and therefore are capable of transferring correspondingly greater amounts of charge. Like other galvanic cells, dry cells may be connected in series to yield batteries with greater voltage outputs, if needed.
Visit this site to learn more about zinc-carbon batteries.
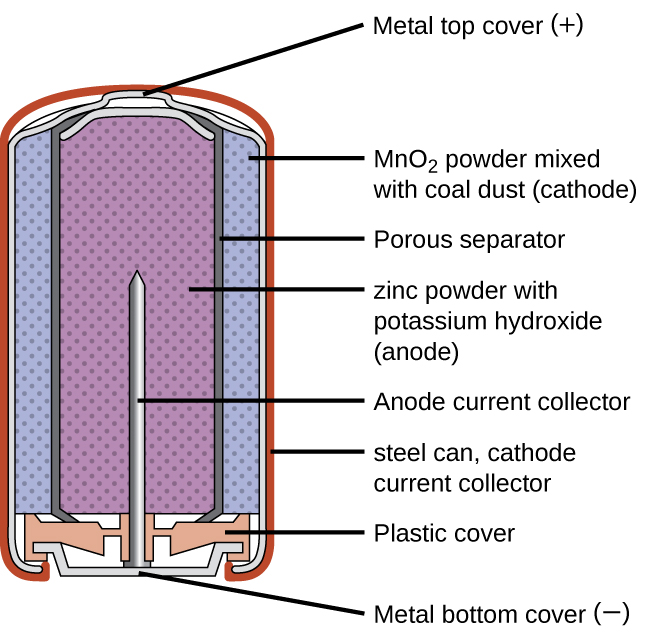
Alkaline batteries ((Figure)) were developed in the 1950s to improve on the performance of the dry cell, and they were designed around the same redox couples. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide. The reactions are
An alkaline battery can deliver about three to five times the energy of a zinc-carbon dry cell of similar size. Alkaline batteries are prone to leaking potassium hydroxide, so they should be removed from devices for long-term storage. While some alkaline batteries are rechargeable, most are not. Attempts to recharge an alkaline battery that is not rechargeable often leads to rupture of the battery and leakage of the potassium hydroxide electrolyte.
Visit this site to learn more about alkaline batteries.
Rechargeable (Secondary) Batteries
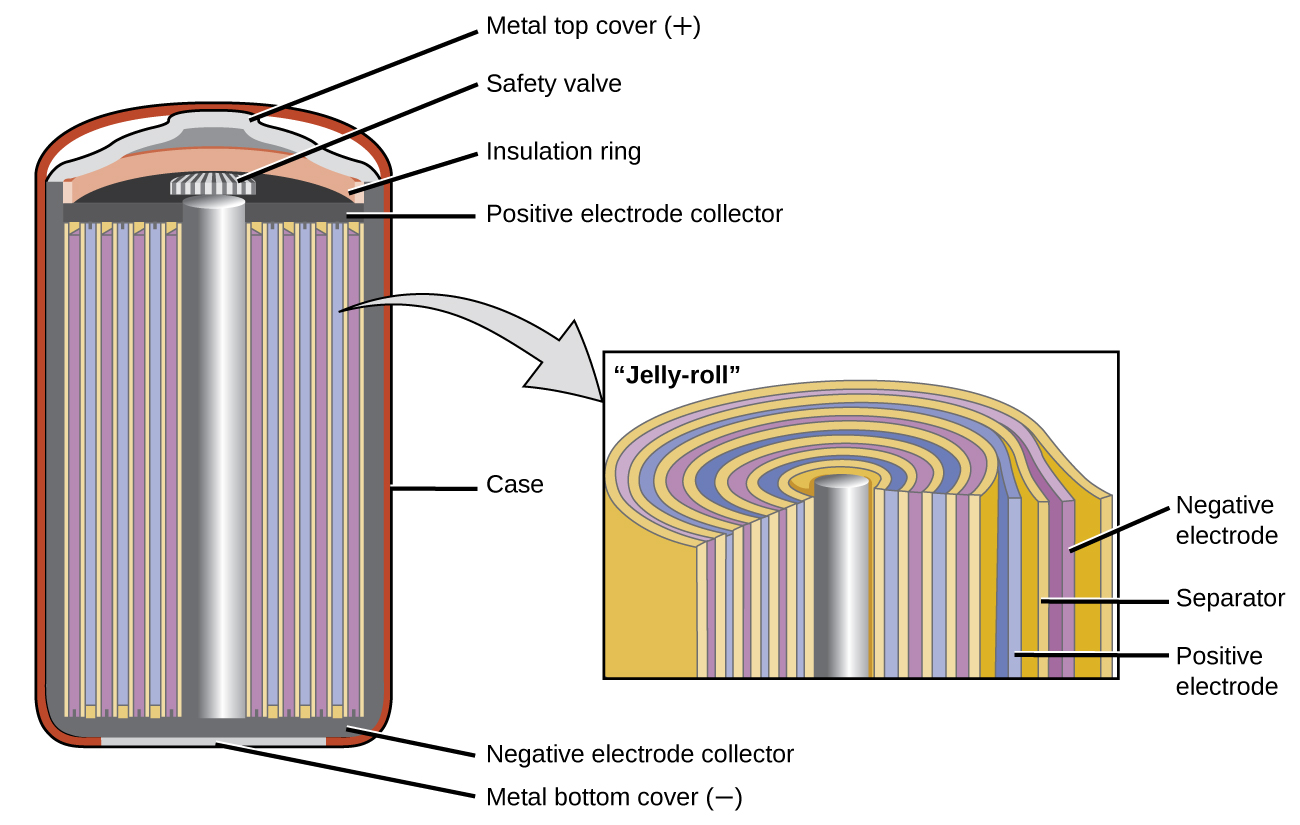
Nickel-cadmium, or NiCd, batteries ((Figure)) consist of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode. The positive and negative plates, which are prevented from shorting by the separator, are rolled together and put into the case. This is a “jelly-roll” design and allows the NiCd cell to deliver much more current than a similar-sized alkaline battery. The reactions are
When properly treated, a NiCd battery can be recharged about 1000 times. Cadmium is a toxic heavy metal so NiCd batteries should never be ruptured or incinerated, and they should be disposed of in accordance with relevant toxic waste guidelines.
Visit this site for more information about nickel cadmium rechargeable batteries.
Lithium ion batteries ((Figure)) are among the most popular rechargeable batteries and are used in many portable electronic devices. The reactions are
The variable stoichiometry of the cell reaction leads to variation in cell voltages, but for typical conditions, x is usually no more than 0.5 and the cell voltage is approximately 3.7 V. Lithium batteries are popular because they can provide a large amount current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.
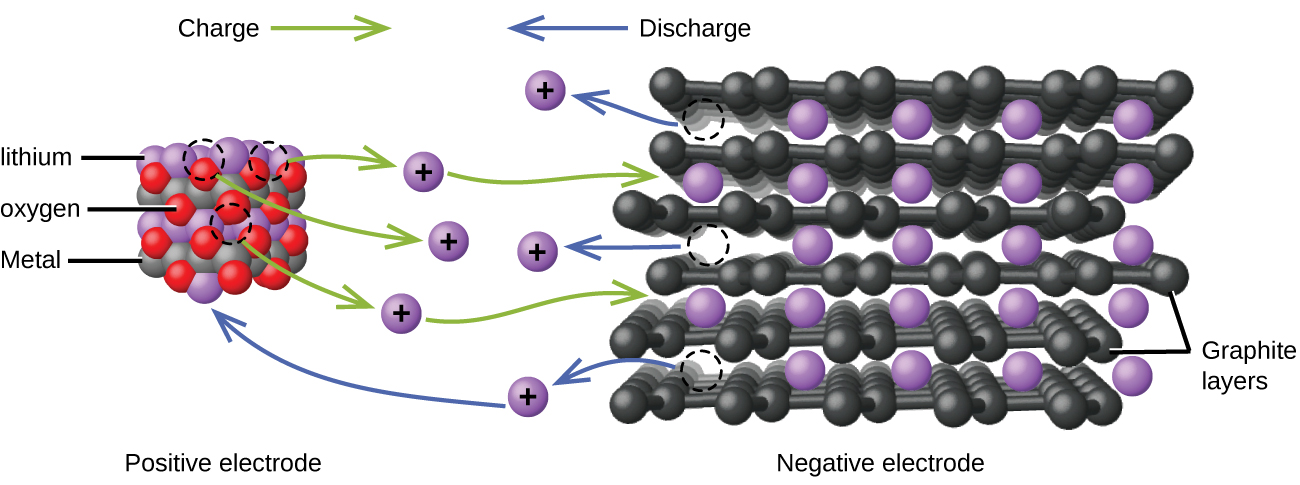
Visit this site for more information about lithium ion batteries.
The lead acid battery ((Figure)) is the type of secondary battery commonly used in automobiles. It is inexpensive and capable of producing the high current required by automobile starter motors. The reactions for a lead acid battery are
Each cell produces 2 V, so six cells are connected in series to produce a 12-V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, H2SO4(aq), but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.
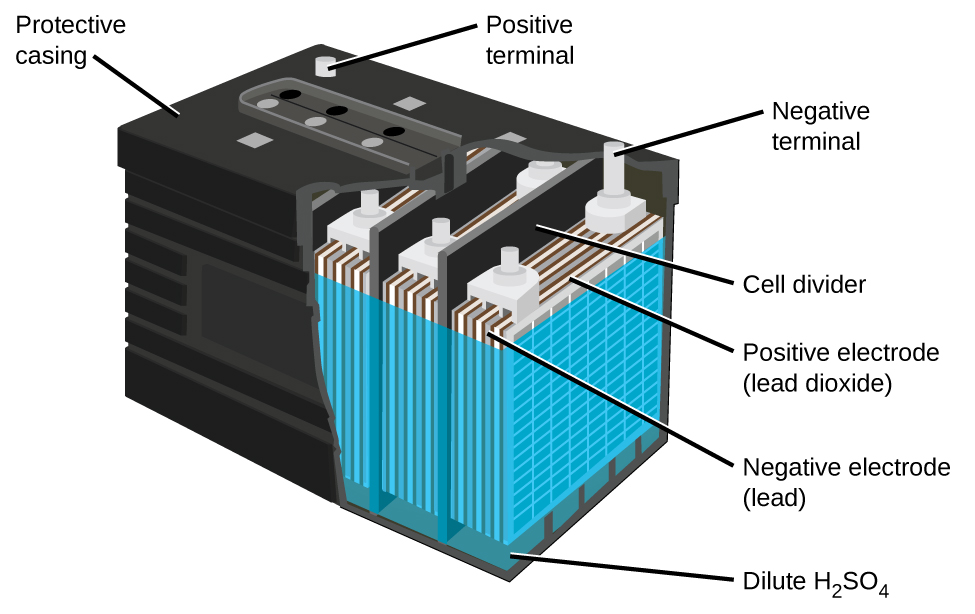
Visit this site for more information about lead acid batteries.
Fuel Cells
A fuel cell is a galvanic cell that uses traditional combustive fuels, most often hydrogen or methane, that are continuously fed into the cell along with an oxidant. (An alternative, but not very popular, name for a fuel cell is a flow battery.) Within the cell, fuel and oxidant undergo the same redox chemistry as when they are combusted, but via a catalyzed electrochemical that is significantly more efficient. For example, a typical hydrogen fuel cell uses graphite electrodes embedded with platinum-based catalysts to accelerate the two half-cell reactions:
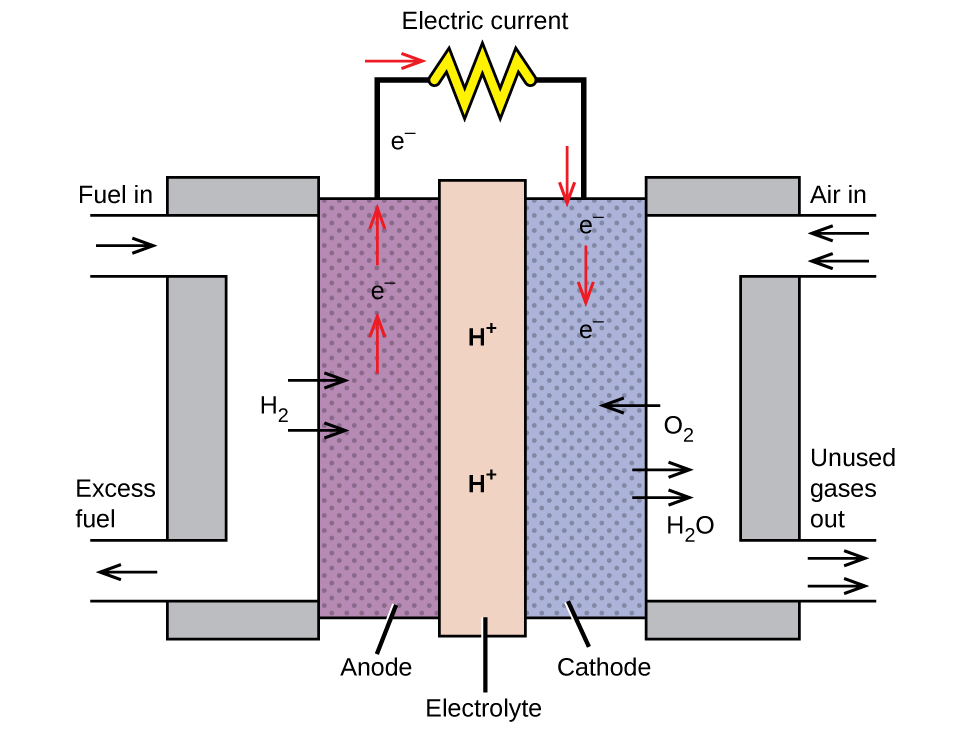
These types of fuel cells generally produce voltages of approximately 1.2 V. Compared to an internal combustion engine, the energy efficiency of a fuel cell using the same redox reaction is typically more than double (~20%–25% for an engine versus ~50%–75% for a fuel cell). Hydrogen fuel cells are commonly used on extended space missions, and prototypes for personal vehicles have been developed, though the technology remains relatively immature.
Check out this link to learn more about fuel cells.
Key Concepts and Summary
Galvanic cells designed specifically to function as electrical power supplies are called batteries. A variety of both single-use batteries (primary cells) and rechargeable batteries (secondary cells) are commercially available to serve a variety of applications, with important specifications including voltage, size, and lifetime. Fuel cells, sometimes called flow batteries, are devices that harness the energy of spontaneous redox reactions normally associated with combustion processes. Like batteries, fuel cells enable the reaction’s electron transfer via an external circuit, but they require continuous input of the redox reactants (fuel and oxidant) from an external reservoir. Fuel cells are typically much more efficient in converting the energy released by the reaction to useful work in comparison to internal combustion engines.
Chemistry End of Chapter Exercises
Consider a battery made from one half-cell that consists of a copper electrode in 1 M CuSO4 solution and another half-cell that consists of a lead electrode in 1 M Pb(NO3)2 solution.
(a) What is the standard cell potential for the battery?
(b) What are the reactions at the anode, cathode, and the overall reaction?
(c) Most devices designed to use dry-cell batteries can operate between 1.0 and 1.5 V. Could this cell be used to make a battery that could replace a dry-cell battery? Why or why not.
(d) Suppose sulfuric acid is added to the half-cell with the lead electrode and some PbSO4(s) forms. Would the cell potential increase, decrease, or remain the same?
Consider a battery with the overall reaction: \(\text{Cu}\left(s\right)+2{\text{Ag}}^{\text{+}}\left(aq\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}\text{2Ag}\left(s\right)+{\text{Cu}}^{2+}\left(aq\right).\)
(a) What is the reaction at the anode and cathode?
(b) A battery is “dead” when its cell potential is zero. What is the value of Q when this battery is dead?
(c) If a particular dead battery was found to have [Cu2+] = 0.11 M, what was the concentration of silver ion?
(a) \(\begin{array}{}\\ \text{anode: Cu}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{\text{Cu}}^{2+}\left(aq\right)+{\text{2e}}^{\text{−}}\phantom{\rule{4em}{0ex}}{E}_{\text{anode}}^{°}=\text{0.34 V}\\ \text{cathode:}\phantom{\rule{0.2em}{0ex}}2\phantom{\rule{0.3em}{0ex}}×\phantom{\rule{0.3em}{0ex}}\left({\text{Ag}}^{\text{+}}\left(aq\right)+{\text{e}}^{\text{−}}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}\text{Ag}\left(s\right)\right)\phantom{\rule{4em}{0ex}}{E}_{\text{cathode}}^{°}=\text{0.7996 V}\end{array};\) (b) 3.5 \(×\) 1015; (c) 5.6 \(×\) 10−9M
Why do batteries go dead, but fuel cells do not?
Batteries are self-contained and have a limited supply of reagents to expend before going dead. Alternatively, battery reaction byproducts accumulate and interfere with the reaction. Because a fuel cell is constantly resupplied with reactants and products are expelled, it can continue to function as long as reagents are supplied.
Use the Nernst equation to explain the drop in voltage observed for some batteries as they discharge.
Using the information thus far in this chapter, explain why battery-powered electronics perform poorly in low temperatures.
Ecell, as described in the Nernst equation, has a term that is directly proportional to temperature. At low temperatures, this term is decreased, resulting in a lower cell voltage provided by the battery to the device—the same effect as a battery running dead.
Glossary
- alkaline battery
- primary battery similar to a dry cell that uses an alkaline (often potassium hydroxide) electrolyte; designed to be an improved replacement for the dry cell, but with more energy storage and less electrolyte leakage than typical dry cell
- battery
- single or series of galvanic cells designed for use as a source of electrical power
- dry cell
- primary battery, also called a zinc-carbon battery, based on the spontaneous oxidation of zinc by manganese(IV)
- fuel cell
- devices similar to galvanic cells that require a continuous feed of redox reactants; also called a flow battery
- lead acid battery
- rechargeable battery commonly used in automobiles; it typically comprises six galvanic cells based on Pb half-reactions in acidic solution
- lithium ion battery
- widely used rechargeable battery commonly used in portable electronic devices, based on lithium ion transfer between the anode and cathode
- nickel-cadmium battery
- rechargeable battery based on Ni/Cd half-cells with applications similar to those of lithium ion batteries
- primary cell
- nonrechargeable battery, suitable for single use only
- secondary cell
- battery designed to allow recharging

