184 Coupled Equilibria
Paul Flowers; Edward J. Neth; William R. Robinson; Klaus Theopold; and Richard Langley
[latexpage]
Learning Objectives
By the end of this section, you will be able to:
- Describe examples of systems involving two (or more) coupled chemical equilibria
- Calculate reactant and product concentrations for coupled equilibrium systems
As discussed in preceding chapters on equilibrium, coupled equilibria involve two or more separate chemical reactions that share one or more reactants or products. This section of this chapter will address solubility equilibria coupled with acid-base and complex-formation reactions.
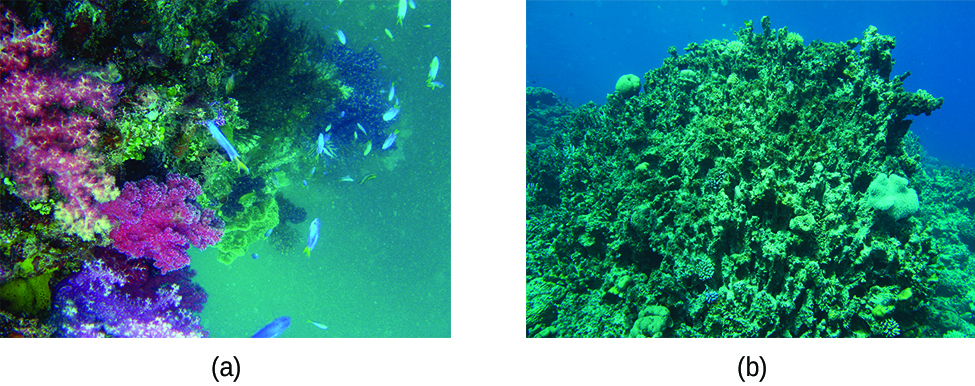
An environmentally relevant example illustrating the coupling of solubility and acid-base equilibria is the impact of ocean acidification on the health of the ocean’s coral reefs. These reefs are built upon skeletons of sparingly soluble calcium carbonate excreted by colonies of corals (small marine invertebrates). The relevant dissolution equilibrium is
Rising concentrations of atmospheric carbon dioxide contribute to an increased acidity of ocean waters due to the dissolution, hydrolysis, and acid ionization of carbon dioxide:
Inspection of these equilibria shows the carbonate ion is involved in the calcium carbonate dissolution and the acid hydrolysis of bicarbonate ion. Combining the dissolution equation with the reverse of the acid hydrolysis equation yields
The equilibrium constant for this net reaction is much greater than the Ksp for calcium carbonate, indicating its solubility is markedly increased in acidic solutions. As rising carbon dioxide levels in the atmosphere increase the acidity of ocean waters, the calcium carbonate skeletons of coral reefs become more prone to dissolution and subsequently less healthy ((Figure)).
Learn more about ocean acidification and how it affects other marine creatures.
This site has detailed information about how ocean acidification specifically affects coral reefs.
The dramatic increase in solubility with increasing acidity described above for calcium carbonate is typical of salts containing basic anions (e.g., carbonate, fluoride, hydroxide, sulfide). Another familiar example is the formation of dental cavities in tooth enamel. The major mineral component of enamel is calcium hydroxyapatite ((Figure)), a sparingly soluble ionic compound whose dissolution equilibrium is

This compound dissolved to yield two different basic ions: triprotic phosphate ions
and monoprotic hydroxide ions:
Of the two basic productions, the hydroxide is, of course, by far the stronger base (it’s the strongest base that can exist in aqueous solution), and so it is the dominant factor providing the compound an acid-dependent solubility. Dental cavities form when the acid waste of bacteria growing on the surface of teeth hastens the dissolution of tooth enamel by reacting completely with the strong base hydroxide, shifting the hydroxyapatite solubility equilibrium to the right. Some toothpastes and mouth rinses contain added NaF or SnF2 that make enamel more acid resistant by replacing the strong base hydroxide with the weak base fluoride:
The weak base fluoride ion reacts only partially with the bacterial acid waste, resulting in a less extensive shift in the solubility equilibrium and an increased resistance to acid dissolution. See the Chemistry in Everyday Life feature on the role of fluoride in preventing tooth decay for more information.
As we saw previously, fluoride ions help protect our teeth by reacting with hydroxylapatite to form fluorapatite, Ca5(PO4)3F. Since it lacks a hydroxide ion, fluorapatite is more resistant to attacks by acids in our mouths and is thus less soluble, protecting our teeth. Scientists discovered that naturally fluorinated water could be beneficial to your teeth, and so it became common practice to add fluoride to drinking water. Toothpastes and mouthwashes also contain amounts of fluoride ((Figure)).

Unfortunately, excess fluoride can negate its advantages. Natural sources of drinking water in various parts of the world have varying concentrations of fluoride, and places where that concentration is high are prone to certain health risks when there is no other source of drinking water. The most serious side effect of excess fluoride is the bone disease, skeletal fluorosis. When excess fluoride is in the body, it can cause the joints to stiffen and the bones to thicken. It can severely impact mobility and can negatively affect the thyroid gland. Skeletal fluorosis is a condition that over 2.7 million people suffer from across the world. So while fluoride can protect our teeth from decay, the US Environmental Protection Agency sets a maximum level of 4 ppm (4 mg/L) of fluoride in drinking water in the US. Fluoride levels in water are not regulated in all countries, so fluorosis is a problem in areas with high levels of fluoride in the groundwater.
The solubility of ionic compounds may also be increased when dissolution is coupled to the formation of a complex ion. For example, aluminum hydroxide dissolves in a solution of sodium hydroxide or another strong base because of the formation of the complex ion \(\text{Al}{\left(\text{OH}\right)}_{4}{}^{\text{−}}.\)
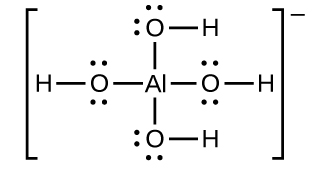
The equations for the dissolution of aluminum hydroxide, the formation of the complex ion, and the combined (net) equation are shown below. As indicated by the relatively large value of K for the net reaction, coupling complex formation with dissolution drastically increases the solubility of Al(OH)3.
Increased Solubility in Acidic Solutions Compute and compare the molar solublities for aluminum hydroxide, Ca(OH)2, dissolved in (a) pure water and (b) a buffer containing 0.100 M acetic acid and 0.100 M sodium acetate.
Solution (a) The molar solubility of calcium fluoride in water is computed considering the dissolution equilibrium only as demonstrated in several previous examples:
(b) The concentration of hydroxide ion of the buffered solution is conveniently calculated by the Henderson-Hasselbalch equation:
At this pH, the concentration of hydroxide ion is
The solubility of Al(OH)3 in this buffer is then calculated from its solubility product expressions:
Compared to pure water, the solubility of aluminum hydroxide in this mildly acidic buffer is approximately ten million times greater (though still relatively low).
Check Your Learning What is the solubility of aluminum hydroxide in a buffer comprised of 0.100 M formic acid and 0.100 M sodium formate?
0.1 M
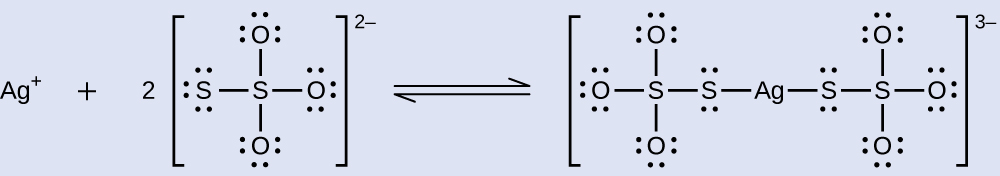
Multiple Equilibria Unexposed silver halides are removed from photographic film when they react with sodium thiosulfate (Na2S2O3, called hypo) to form the complex ion \(\text{Ag}{\left({\text{S}}_{2}{\text{O}}_{3}\right)}_{2}{}^{\text{3−}}\) (Kf = 4.7 \(×\) 1013).
What mass of Na2S2O3 is required to prepare 1.00 L of a solution that will dissolve 1.00 g of AgBr by the formation of \(\text{Ag}{\left({\text{S}}_{2}{\text{O}}_{3}\right)}_{2}{}^{\text{3−}}?\)
Solution Two equilibria are involved when AgBr dissolves in a solution containing the \({\text{S}}_{2}{\text{O}}_{3}{}^{\text{2−}}\) ion:
dissolution: \(\text{AgBr}\left(s\right)⇌{\text{Ag}}^{\text{+}}\left(aq\right)+{\text{Br}}^{\text{−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=5.0\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−13}}\)
complexation: \({\text{Ag}}^{\text{+}}\left(aq\right)+{\text{2S}}_{2}{\text{O}}_{3}{}^{\text{2−}}\left(aq\right)⇌\text{Ag}{\left({\text{S}}_{2}{\text{O}}_{3}\right)}_{2}{}^{\text{3−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{f}}=4.7\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{13}\)
First, calculate the concentration of bromide that will result when the 1.00 g of AgBr is completely dissolved via the cited complexation reaction:
Next, use this bromide molarity and the solubility product for silver bromide to calculate the silver ion molarity in the solution:
Based on the stoichiometry of the complex ion formation, the concentration of complex ion produced is
Use the silver ion and complex ion concentrations and the formation constant for the complex ion to compute the concentration of thiosulfate ion.
Finally, use this molar concentration to derive the required mass of sodium thiosulfate:
Thus, 1.00 L of a solution prepared from 1.7 g Na2S2O3 dissolves 1.0 g of AgBr.
Check Your LearningAgCl(s), silver chloride, has a very low solubility: \(\text{AgCl}\left(s\right)⇌{\text{Ag}}^{\text{+}}\left(aq\right)+{\text{Cl}}^{\text{−}}\left(aq\right),\)Ksp = 1.6 \(×\) 10–10. Adding ammonia significantly increases the solubility of AgCl because a complex ion is formed: \({\text{Ag}}^{\text{+}}\left(aq\right)+2{\text{NH}}_{3}\left(aq\right)⇌\text{Ag}{\left({\text{NH}}_{3}\right)}_{2}{}^{+}\left(aq\right),\) Kf = 1.7 \(×\) 107. What mass of NH3 is required to prepare 1.00 L of solution that will dissolve 2.00 g of AgCl by formation of \(\text{Ag}{\left({\text{NH}}_{3}\right)}_{2}{}^{\text{+}}?\)
1.00 L of a solution prepared with 4.81 g NH3 dissolves 2.0 g of AgCl.
Key Concepts and Summary
Systems involving two or more chemical equilibria that share one or more reactant or product are called coupled equilibria. Common examples of coupled equilibria include the increased solubility of some compounds in acidic solutions (coupled dissolution and neutralization equilibria) and in solutions containing ligands (coupled dissolution and complex formation). The equilibrium tools from other chapters may be applied to describe and perform calculations on these systems.
Chemistry End of Chapter Exercises
A saturated solution of a slightly soluble electrolyte in contact with some of the solid electrolyte is said to be a system in equilibrium. Explain. Why is such a system called a heterogeneous equilibrium?
Calculate the equilibrium concentration of Ni2+ in a 1.0-M solution [Ni(NH3)6](NO3)2.
0.014 M
Calculate the equilibrium concentration of Zn2+ in a 0.30-M solution of \(\text{Zn}{\left(\text{CN}\right)}_{4}{}^{\text{2−}}.\)
Calculate the equilibrium concentration of Cu2+ in a solution initially with 0.050 M Cu2+ and 1.00 M NH3.
7.2 \(×\) 10–15M
Calculate the equilibrium concentration of Zn2+ in a solution initially with 0.150 M Zn2+ and 2.50 M CN–.
Calculate the Fe3+ equilibrium concentration when 0.0888 mole of K3[Fe(CN)6] is added to a solution with 0.0.00010 M CN–.
4.4 \(×\) 10−22M
Calculate the Co2+ equilibrium concentration when 0.100 mole of [Co(NH3)6](NO3)2 is added to a solution with 0.025 M NH3. Assume the volume is 1.00 L.
Calculate the molar solubility of Sn(OH)2 in a buffer solution containing equal concentrations of NH3 and \({\text{NH}}_{4}{}^{+}.\)
Calculate the molar solubility of Al(OH)3 in a buffer solution with 0.100 M NH3 and 0.400 M \({\text{NH}}_{4}{}^{+}.\)
[OH−] = 4.5 \(×\) 10−5; [Al3+] = 2.2 \(×\) 10–20 (molar solubility)
What is the molar solubility of CaF2 in a 0.100-M solution of HF? Ka for HF = 6.4 \(×\) 10–4.
What is the molar solubility of BaSO4 in a 0.250-M solution of NaHSO4? Ka for \({\text{HSO}}_{4}{}^{\text{−}}\) = 1.2 \(×\) 10–2.
\(\left[{\text{SO}}_{4}{}^{\text{2−}}\right]=0.049\phantom{\rule{0.2em}{0ex}}M\); [Ba2+] = 4.7 \(×\) 10–7 (molar solubility)
What is the molar solubility of Tl(OH)3 in a 0.10-M solution of NH3?
What is the molar solubility of Pb(OH)2 in a 0.138-M solution of CH3NH2?
[OH–] = 7.6 \(×\) 10−3M; [Pb2+] = 2.1 \(×\) 10–11 (molar solubility)
A solution of 0.075 M CoBr2 is saturated with H2S ([H2S] = 0.10 M). What is the minimum pH at which CoS begins to precipitate?
\(\text{CoS}\left(s\right)⇌{\text{Co}}^{\text{2+}}\left(aq\right)+{\text{S}}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=4.5\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−27}}\)
\({\text{H}}_{2}\text{S}\left(aq\right)+2{\text{H}}_{2}\text{O}\left(l\right)⇌2{\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{S}}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}K=1.0\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−26}}\)
A 0.125-M solution of Mn(NO3)2 is saturated with H2S ([H2S] = 0.10 M). At what pH does MnS begin to precipitate?
\(\text{MnS}\left(s\right)⇌{\text{Mn}}^{\text{2+}}\left(aq\right)+{\text{S}}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=4.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−22}}\)
\({\text{H}}_{2}\text{S}\left(aq\right)+2{\text{H}}_{2}\text{O}\left(l\right)⇌2{\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{S}}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}K=1.0\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−26}}\)
7.66
Both AgCl and AgI dissolve in NH3.
(a) What mass of AgI dissolves in 1.0 L of 1.0 M NH3?
(b) What mass of AgCl dissolves in 1.0 L of 1.0 M NH3?
The following question is taken from a Chemistry Advanced Placement Examination and is used with the permission of the Educational Testing Service.
Solve the following problem:
\({\text{MgF}}_{2}\left(s\right)⇌{\text{Mg}}^{\text{2+}}\left(aq\right)+2{\text{F}}^{\text{−}}\left(aq\right)\)
In a saturated solution of MgF2 at 18 °C, the concentration of Mg2+ is 1.21 \(×\) 10–3M. The equilibrium is represented by the preceding equation.
(a) Write the expression for the solubility-product constant, Ksp, and calculate its value at 18 °C.
(b) Calculate the equilibrium concentration of Mg2+ in 1.000 L of saturated MgF2 solution at 18 °C to which 0.100 mol of solid KF has been added. The KF dissolves completely. Assume the volume change is negligible.
(c) Predict whether a precipitate of MgF2 will form when 100.0 mL of a 3.00 \(×\) 10–3–M solution of Mg(NO3)2 is mixed with 200.0 mL of a 2.00 \(×\) 10–3–M solution of NaF at 18 °C. Show the calculations to support your prediction.
(d) At 27 °C the concentration of Mg2+ in a saturated solution of MgF2 is 1.17 \(×\) 10–3M. Is the dissolving of MgF2 in water an endothermic or an exothermic process? Give an explanation to support your conclusion.
(a) Ksp = [Mg2+][F–]2 = (1.21 \(×\) 10–3)(2 \(×\) 1.21 \(×\) 10–3)2 = 7.09 \(×\) 10–9
(b) 7.09 \(×\) 10–7M
(c) Determine the concentration of Mg2+ and F– that will be present in the final volume. Compare the value of the ion product [Mg2+][F–]2 with Ksp. If this value is larger than Ksp, precipitation will occur.
0.1000 L \(×\) 3.00 \(×\) 10–3M Mg(NO3)2 = 0.3000 L \(×\) M Mg(NO3)2
M Mg(NO3)2 = 1.00 \(×\) 10–3M
0.2000 L \(×\) 2.00 \(×\) 10–3M NaF = 0.3000 L \(×\) M NaF
M NaF = 1.33 \(×\) 10–3M
ion product = (1.00 \(×\) 10–3)(1.33 \(×\) 10–3)2 = 1.77 \(×\) 10–9 This value is smaller than Ksp, so no precipitation will occur.
(d) MgF2 is less soluble at 27 °C than at 18 °C. Because added heat acts like an added reagent, when it appears on the product side, the Le Châtelier’s principle states that the equilibrium will shift to the reactants’ side to counter the stress. Consequently, less reagent will dissolve. This situation is found in our case. Therefore, the reaction is exothermic.
Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility greater than in pure water: CuCl, CaCO3, MnS, PbBr2, CaF2? Explain your answer.
Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility greater than in pure water: AgBr, BaF2, Ca3(PO4)2, ZnS, PbI2? Explain your answer.
BaF2, Ca3(PO4)2, ZnS; each is a salt of a weak acid, and the \(\left[{\text{H}}_{3}{\text{O}}^{+}\right]\) from perchloric acid reduces the equilibrium concentration of the anion, thereby increasing the concentration of the cations
What is the effect on the amount of solid Mg(OH)2 that dissolves and the concentrations of Mg2+ and OH– when each of the following are added to a mixture of solid Mg(OH)2 and water at equilibrium?
(a) MgCl2
(b) KOH
(c) HClO4
(d) NaNO3
(e) Mg(OH)2
What is the effect on the amount of CaHPO4 that dissolves and the concentrations of Ca2+ and \({\text{HPO}}_{4}{}^{\text{−}}\) when each of the following are added to a mixture of solid CaHPO4 and water at equilibrium?
(a) CaCl2
(b) HCl
(c) KClO4
(d) NaOH
(e) CaHPO4
Effect on amount of solid CaHPO4, [Ca2+], [OH–]: (a) increase, increase, decrease; (b) decrease, increase, decrease; (c) no effect, no effect, no effect; (d) decrease, increase, decrease; (e) increase, no effect, no effect
Identify all chemical species present in an aqueous solution of Ca3(PO4)2 and list these species in decreasing order of their concentrations. (Hint: Remember that the \({\text{PO}}_{4}{}^{\text{3−}}\) ion is a weak base.)
Glossary
- coupled equilibria
- system characterized the simultaneous establishment of two or more equilibrium reactions sharing one or more reactant or product

