159 Electrolytes
Paul Flowers; Edward J. Neth; William R. Robinson; Klaus Theopold; and Richard Langley
[latexpage]
Learning Objectives
By the end of this module, you will be able to:
- Define and give examples of electrolytes
- Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes
- Relate electrolyte strength to solute-solvent attractive forces
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit ((Figure)).
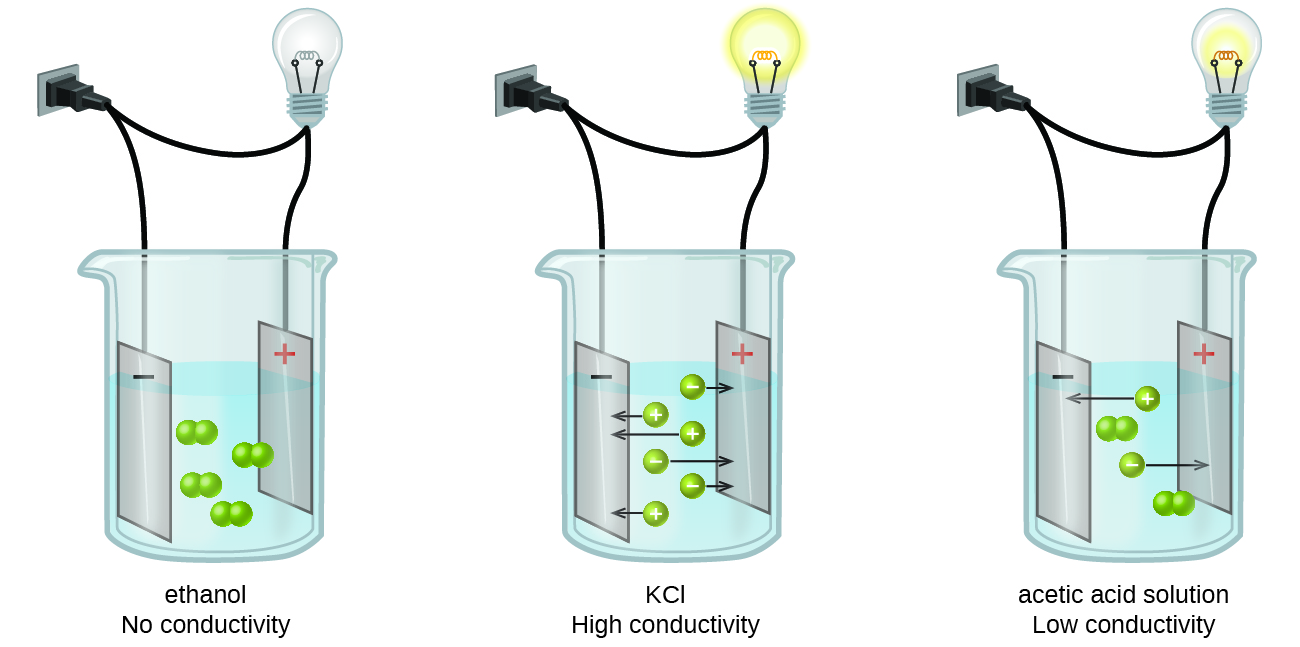
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in (Figure). The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water.
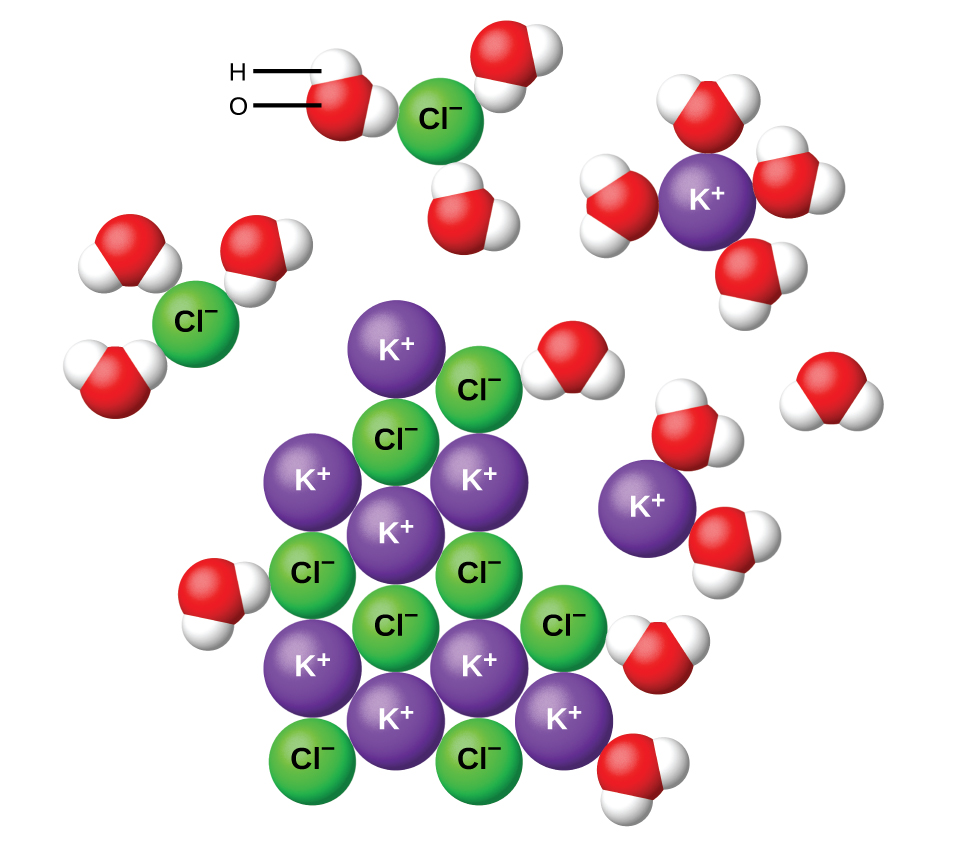
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. Even sparingly, soluble ionic compounds are strong electrolytes, since the small amount that does dissolve will dissociate completely.
Consider what happens at the microscopic level when solid KCl is added to water. Ion-dipole forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules surround individual K+ and Cl− ions, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as (Figure) shows. Overcoming the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution as the ions transition from fixed positions in the undissolved compound to widely dispersed, solvated ions in solution.
Covalent Electrolytes
Pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25 °C. Water ionizes when one molecule of water gives up a proton (H+ ion) to another molecule of water, yielding hydronium and hydroxide ions.
In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl molecules. This gas contains no ions. However, an aqueous solution of HCl is a very good conductor, indicating that an appreciable concentration of ions exists within the solution.
Because HCl is an acid, its molecules react with water, transferring H+ ions to form hydronium ions (H3O+) and chloride ions (Cl−):
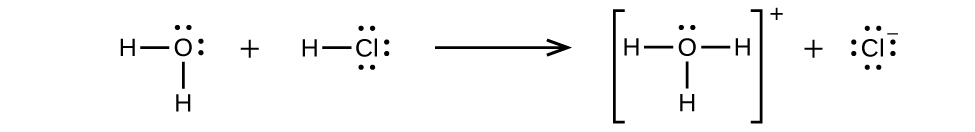
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.
Key Concepts and Summary
Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Dissolution of an ionic compound is facilitated by ion-dipole attractions between the ions of the compound and the polar water molecules. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to only a small extent and are weak electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in water.
Chemistry End of Chapter Exercises
Explain why the ions Na+ and Cl− are strongly solvated in water but not in hexane, a solvent composed of nonpolar molecules.
Crystals of NaCl dissolve in water, a polar liquid with a very large dipole moment, and the individual ions become strongly solvated. Hexane is a nonpolar liquid with a dipole moment of zero and, therefore, does not significantly interact with the ions of the NaCl crystals.
Explain why solutions of HBr in benzene (a nonpolar solvent) are nonconductive, while solutions in water (a polar solvent) are conductive.
Consider the solutions presented:
(a) Which of the following sketches best represents the ions in a solution of Fe(NO3)3(aq)?
3_img.jpg#fixme#fixme)
(b) Write a balanced chemical equation showing the products of the dissolution of Fe(NO3)3.
(a) Fe(NO3)3 is a strong electrolyte, thus it should completely dissociate into Fe3+ and \({\text{NO}}_{3}{}^{\text{−}}\) ions. Therefore, (z) best represents the solution. (b) \(\text{Fe}{\left({\text{NO}}_{3}\right)}_{3}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{\text{Fe}}^{\text{3+}}\left(aq\right)+3{\text{NO}}_{3}{}^{\text{−}}\left(aq\right)\)
Compare the processes that occur when methanol (CH3OH), hydrogen chloride (HCl), and sodium hydroxide (NaOH) dissolve in water. Write equations and prepare sketches showing the form in which each of these compounds is present in its respective solution.
What is the expected electrical conductivity of the following solutions?
(a) NaOH(aq)
(b) HCl(aq)
(c) C6H12O6(aq) (glucose)
(d) NH3(aq)
(a) high conductivity (solute is an ionic compound that will dissociate when dissolved); (b) high conductivity (solute is a strong acid and will ionize completely when dissolved); (c) nonconductive (solute is a covalent compound, neither acid nor base, unreactive towards water); (d) low conductivity (solute is a weak base and will partially ionize when dissolved)
Why are most solid ionic compounds electrically nonconductive, whereas aqueous solutions of ionic compounds are good conductors? Would you expect a liquid (molten) ionic compound to be electrically conductive or nonconductive? Explain.
Indicate the most important type of intermolecular attraction responsible for solvation in each of the following solutions:
(a) the solutions in (Figure)
(b) methanol, CH3OH, dissolved in ethanol, C2H5OH
(c) methane, CH4, dissolved in benzene, C6H6
(d) the polar halocarbon CF2Cl2 dissolved in the polar halocarbon CF2ClCFCl2
(e) O2(l) in N2(l)
(a) ion-dipole; (b) hydrogen bonds; (c) dispersion forces; (d) dipole-dipole attractions; (e) dispersion forces
Glossary
- dissociation
- physical process accompanying the dissolution of an ionic compound in which the compound’s constituent ions are solvated and dispersed throughout the solution
- electrolyte
- substance that produces ions when dissolved in water
- ion-dipole attraction
- electrostatic attraction between an ion and a polar molecule
- nonelectrolyte
- substance that does not produce ions when dissolved in water
- strong electrolyte
- substance that dissociates or ionizes completely when dissolved in water
- weak electrolyte
- substance that ionizes only partially when dissolved in water

