182 Precipitation and Dissolution
Paul Flowers; Edward J. Neth; William R. Robinson; Klaus Theopold; and Richard Langley
[latexpage]
Learning Objectives
By the end of this section, you will be able to:
- Write chemical equations and equilibrium expressions representing solubility equilibria
- Carry out equilibrium computations involving solubility, equilibrium expressions, and solute concentrations
Solubility equilibria are established when the dissolution and precipitation of a solute species occur at equal rates. These equilibria underlie many natural and technological processes, ranging from tooth decay to water purification. An understanding of the factors affecting compound solubility is, therefore, essential to the effective management of these processes. This section applies previously introduced equilibrium concepts and tools to systems involving dissolution and precipitation.
The Solubility Product
Recall from the chapter on solutions that the solubility of a substance can vary from essentially zero (insoluble or sparingly soluble) to infinity (miscible). A solute with finite solubility can yield a saturated solution when it is added to a solvent in an amount exceeding its solubility, resulting in a heterogeneous mixture of the saturated solution and the excess, undissolved solute. For example, a saturated solution of silver chloride is one in which the equilibrium shown below has been established.
In this solution, an excess of solid AgCl dissolves and dissociates to produce aqueous Ag+ and Cl– ions at the same rate that these aqueous ions combine and precipitate to form solid AgCl ((Figure)). Because silver chloride is a sparingly soluble salt, the equilibrium concentration of its dissolved ions in the solution is relatively low.
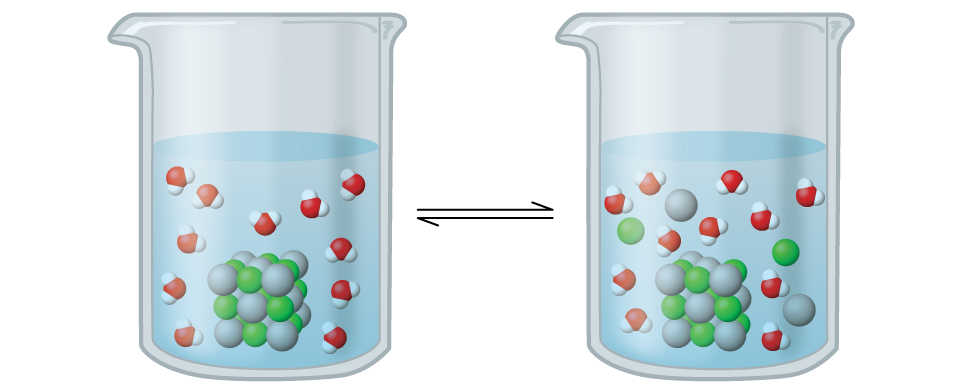
The equilibrium constant for solubility equilibria such as this one is called the solubility product constant, Ksp, in this case
Recall that only gases and solutes are represented in equilibrium constant expressions, so the Ksp does not include a term for the undissolved AgCl. A listing of solubility product constants for several sparingly soluble compounds is provided in Appendix J.
Writing Equations and Solubility Products Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds:
(a) AgI, silver iodide, a solid with antiseptic properties
(b) CaCO3, calcium carbonate, the active ingredient in many over-the-counter chewable antacids
(c) Mg(OH)2, magnesium hydroxide, the active ingredient in Milk of Magnesia
(d) Mg(NH4)PO4, magnesium ammonium phosphate, an essentially insoluble substance used in tests for magnesium
(e) Ca5(PO4)3OH, the mineral apatite, a source of phosphate for fertilizers
Solution\(\begin{array}{ccccc}\left(\text{a}\right)\phantom{\rule{0.2em}{0ex}}\text{AgI}\left(s\right)⇌{\text{Ag}}^{\text{+}}\left(aq\right)+{\text{I}}^{\text{−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & {\left[\text{Ag}}^{\text{+}}\right]\left[{\text{I}}^{\text{−}}\right]\hfill \\ \left(\text{b}\right)\phantom{\rule{0.2em}{0ex}}{\text{CaCO}}_{3}\left(s\right)⇌{\text{Ca}}^{\text{2+}}\left(aq\right)+{\text{CO}}_{3}{}^{\text{2−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Ca}}^{\text{2+}}{\right]\left[\text{CO}}_{3}{}^{\text{2−}}\right]\hfill \\ \left(\text{c}\right)\phantom{\rule{0.2em}{0ex}}{\text{Mg}\left(\text{OH}\right)}_{2}\left(s\right)⇌{\text{Mg}}^{\text{2+}}\left(aq\right)+{\text{2OH}}^{\text{−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Mg}}^{\text{2+}}\right]{\left[{\text{OH}}^{\text{−}}\right]}^{2}\hfill \\ \left(\text{d}\right)\phantom{\rule{0.2em}{0ex}}\text{Mg}\left({\text{NH}}_{\text{4}}\right){\text{PO}}_{\text{4}}\left(s\right)⇌{\text{Mg}}^{\text{2+}}\left(aq\right)+{\text{NH}}_{4}{}^{\text{+}}\left(aq\right)+{\text{PO}}_{4}{}^{\text{3−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Mg}}^{\text{2+}}{\right]\left[\text{NH}}_{4}{}^{\text{+}}\right]{\left[\text{PO}}_{4}{}^{\text{3−}}\right]\hfill \\ \left(\text{e}\right)\phantom{\rule{0.2em}{0ex}}{\text{Ca}}_{5}\left({\text{PO}}_{4}\right)3\text{OH}\left(s\right)⇌{\text{5Ca}}^{\text{2+}}\left(aq\right)+{\text{3PO}}_{4}{}^{\text{3−}}\left(aq\right)+{\text{OH}}^{\text{−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & {{\left[\text{Ca}}^{\text{2+}}\right]}^{5}\left[\text{P}{\text{O}}_{4}{}^{\text{3−}}{\right]}^{3}\left[{\text{OH}}^{\text{−}}\right]\hfill \end{array}\)
Check Your LearningWrite the dissolution equation and the solubility product for each of the following slightly soluble compounds:
(a) BaSO4
(b) Ag2SO4
(c) Al(OH)3
(d) Pb(OH)Cl
\(\begin{array}{ccccc}\left(\text{a}\right)\phantom{\rule{0.2em}{0ex}}{\text{BaSO}}_{4}\left(s\right)⇌{\text{Ba}}^{\text{2+}}\left(aq\right)+{\text{SO}}_{4}{}^{\text{2−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Ba}}^{\text{2+}}{\right]\left[\text{SO}}_{4}{{}^{2}}^{\text{−}}\right];\hfill \\ \left(\text{b}\right)\phantom{\rule{0.2em}{0ex}}{\text{Ag}}_{2}{\text{SO}}_{4}\left(s\right)⇌{\text{2Ag}}^{\text{+}}\left(aq\right)+{\text{SO}}_{4}{}^{\text{2−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & {\left[{\text{Ag}}^{\text{+}}\right]}^{2}\left[{\text{SO}}_{4}{}^{\text{2−}}\right];\hfill \\ \left(\text{c}\right)\phantom{\rule{0.2em}{0ex}}{\text{Al}\left(\text{OH}\right)}_{3}\left(s\right)⇌{\text{Al}}^{\text{3+}}\left(aq\right)+{\text{3OH}}^{\text{−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Al}}^{\text{3+}}\right]{\left[\text{OH}}^{\text{−}}{\right]}^{\text{3}};\hfill \\ \left(\text{d}\right)\phantom{\rule{0.2em}{0ex}}\text{Pb(OH)Cl(}s\right)⇌{\text{Pb}}^{\text{2+}}\left(aq\right)+{\text{OH}}^{\text{−}}\left(aq\right)+{\text{Cl}}^{\text{−}}\left(aq\right)\hfill & & \hfill {K}_{\text{sp}}& =\hfill & \left[{\text{Pb}}^{\text{2+}}\right]{\left[\text{OH}}^{\text{−}}\right]\left[{\text{Cl}}^{\text{−}}\right]\hfill \end{array}\)
Ksp and Solubility
The Ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process involves only dissociation and solvation, for example:
For cases such as these, one may derive Ksp values from provided solubilities, or vice-versa. Calculations of this sort are most conveniently performed using a compound’s molar solubility, measured as moles of dissolved solute per liter of saturated solution.
Calculation of Ksp from Equilibrium Concentrations Fluorite, CaF2, is a slightly soluble solid that dissolves according to the equation:
The concentration of Ca2+ in a saturated solution of CaF2 is 2.15 \(×\) 10–4M. What is the solubility product of fluorite?
Solution According to the stoichiometry of the dissolution equation, the fluoride ion molarity of a CaF2 solution is equal to twice its calcium ion molarity:
Substituting the ion concentrations into the Ksp expression gives
Check Your LearningIn a saturated solution of Mg(OH)2, the concentration of Mg2+ is 1.31 \(×\) 10–4M. What is the solubility product for Mg(OH)2?
8.99 \(×\) 10–12
Determination of Molar Solubility from Ksp The Ksp of copper(I) bromide, CuBr, is 6.3 \(×\) 10–9. Calculate the molar solubility of copper bromide.
SolutionThe dissolution equation and solubility product expression are
Following the ICE approach to this calculation yields the table
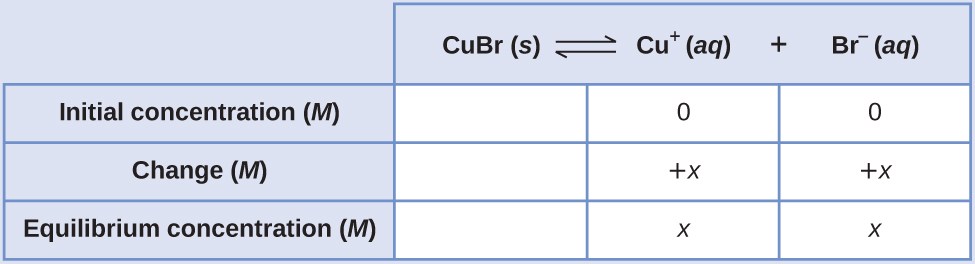
Substituting the equilibrium concentration terms into the solubility product expression and solving for x yields
Since the dissolution stoichiometry shows one mole of copper(I) ion and one mole of bromide ion are produced for each moles of Br dissolved, the molar solubility of CuBr is 7.9 \(×\) 10–5M.
Check Your Learning The Ksp of AgI is 1.5 \(×\) 10–16. Calculate the molar solubility of silver iodide.
1.2 \(×\) 10–8M
Determination of Molar Solubility from KspThe Ksp of calcium hydroxide, Ca(OH)2, is 1.3 \(×\) 10–6. Calculate the molar solubility of calcium hydroxide.
SolutionThe dissolution equation and solubility product expression are
The ICE table for this system is
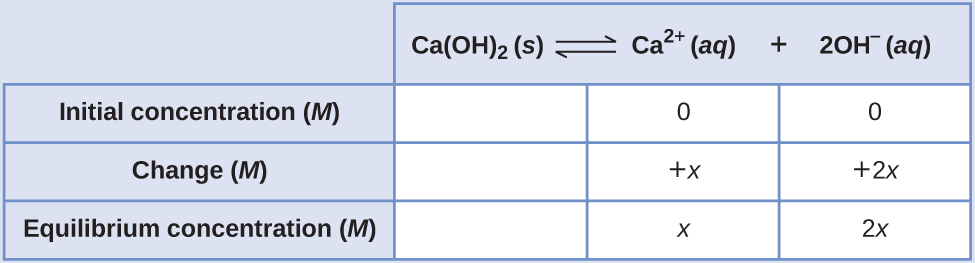
Substituting terms for the equilibrium concentrations into the solubility product expression and solving for x gives
As defined in the ICE table, x is the molarity of calcium ion in the saturated solution. The dissolution stoichiometry shows a 1:1 relation between moles of calcium ion in solution and moles of compound dissolved, and so, the molar solubility of Ca(OH)2 is 6.9 \(×\) 10–3M.
Check Your Learning The Ksp of PbI2 is 1.4 \(×\) 10–8. Calculate the molar solubility of lead(II) iodide.
1.5 \(×\) 10–3M
Determination of Ksp from Gram SolubilityMany of the pigments used by artists in oil-based paints ((Figure)) are sparingly soluble in water. For example, the solubility of the artist’s pigment chrome yellow, PbCrO4, is 4.6 \(×\) 10–6 g/L. Determine the solubility product for PbCrO4.

Solution
Before calculating the solubility product, the provided solubility must be converted to molarity:
The dissolution equation for this compound is
The dissolution stoichiometry shows a 1:1 relation between the molar amounts of compound and its two ions, and so both [Pb2+] and \(\left[{\text{CrO}}_{4}{}^{\text{2−}}\right]\) are equal to the molar solubility of PbCrO4:
Ksp = [Pb2+]\(\left[{\text{CrO}}_{4}{}^{\text{2−}}\right]\) = (1.4 \(×\) 10–8)(1.4 \(×\) 10–8) = 2.0 \(×\) 10–16
Check Your LearningThe solubility of TlCl [thallium(I) chloride], an intermediate formed when thallium is being isolated from ores, is 3.12 grams per liter at 20 °C. What is its solubility product?
2.08 \(×\) 10–4
Calculating the Solubility of Hg2Cl2 Calomel, Hg2Cl2, is a compound composed of the diatomic ion of mercury(I), \({\text{Hg}}_{2}{}^{\text{2+}},\) and chloride ions, Cl–. Although most mercury compounds are now known to be poisonous, eighteenth-century physicians used calomel as a medication. Their patients rarely suffered any mercury poisoning from the treatments because calomel has a very low solubility, as suggested by its very small Ksp:
Calculate the molar solubility of Hg2Cl2.
SolutionThe dissolution stoichiometry shows a 1:1 relation between the amount of compound dissolved and the amount of mercury(I) ions, and so the molar solubility of Hg2Cl2 is equal to the concentration of \({\text{Hg}}_{2}{}^{\text{2+}}\) ions
Following the ICE approach results in
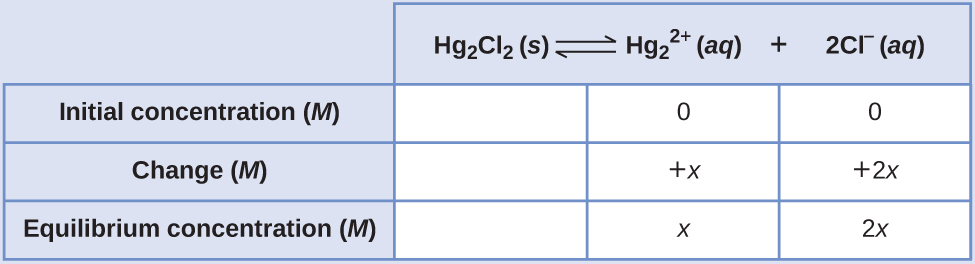
Substituting the equilibrium concentration terms into the solubility product expression and solving for x gives
The dissolution stoichiometry shows the molar solubility of Hg2Cl2 is equal to \(\left[{\text{Hg}}_{\text{2}}{}^{\text{2+}}\right],\) or 6.5 \(×\) 10–7M.
Check Your LearningDetermine the molar solubility of MgF2 from its solubility product: Ksp = 6.4 \(×\) 10–9.
1.2 \(×\) 10–3M
Various types of medical imaging techniques are used to aid diagnoses of illnesses in a noninvasive manner. One such technique utilizes the ingestion of a barium compound before taking an X-ray image. A suspension of barium sulfate, a chalky powder, is ingested by the patient. Since the Ksp of barium sulfate is 2.3 \(×\) 10–8, very little of it dissolves as it coats the lining of the patient’s intestinal tract. Barium-coated areas of the digestive tract then appear on an X-ray as white, allowing for greater visual detail than a traditional X-ray ((Figure)).
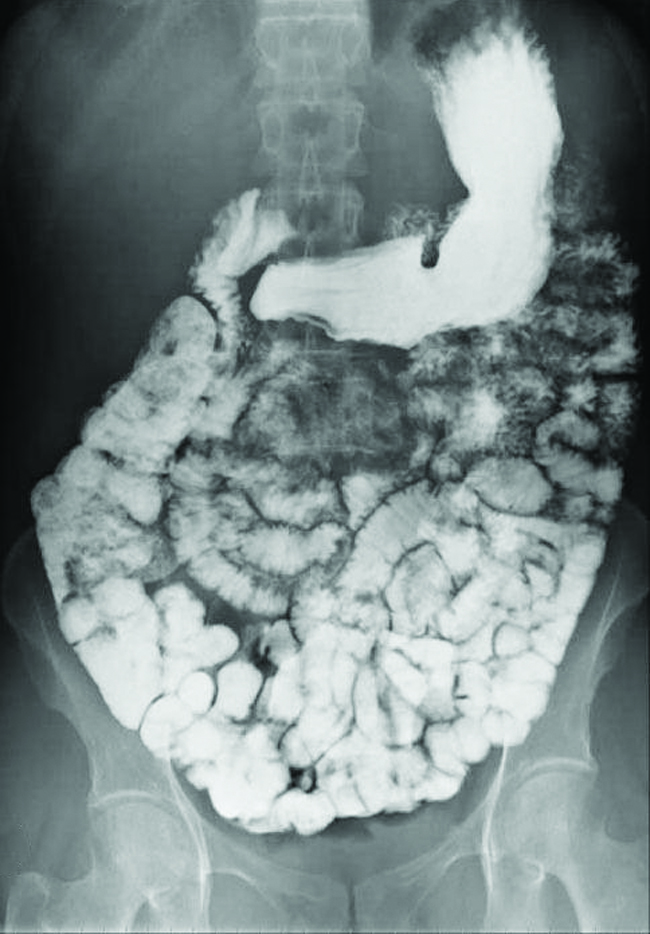
Medical imaging using barium sulfate can be used to diagnose acid reflux disease, Crohn’s disease, and ulcers in addition to other conditions.
Visit this website for more information on how barium is used in medical diagnoses and which conditions it is used to diagnose.
Predicting Precipitation
The equation that describes the equilibrium between solid calcium carbonate and its solvated ions is:
It is important to realize that this equilibrium is established in any aqueous solution containing Ca2+ and CO32– ions, not just in a solution formed by saturating water with calcium carbonate. Consider, for example, mixing aqueous solutions of the soluble compounds sodium carbonate and calcium nitrate. If the concentrations of calcium and carbonate ions in the mixture do not yield a reaction quotient, Qsp, that exceeds the solubility product, Ksp, then no precipitation will occur. If the ion concentrations yield a reaction quotient greater than the solubility product, then precipitation will occur, lowering those concentrations until equilibrium is established (Qsp = Ksp). The comparison of Qsp to Ksp to predict precipitation is an example of the general approach to predicting the direction of a reaction first introduced in the chapter on equilibrium. For the specific case of solubility equilibria:
Qsp < Ksp: the reaction proceeds in the forward direction (solution is not saturated; no precipitation observed)
Qsp > Ksp: the reaction proceeds in the reverse direction (solution is supersaturated; precipitation will occur)
This predictive strategy and related calculations are demonstrated in the next few example exercises.
Precipitation of Mg(OH)2 The first step in the preparation of magnesium metal is the precipitation of Mg(OH)2 from sea water by the addition of lime, Ca(OH)2, a readily available inexpensive source of OH– ion:
The concentration of Mg2+(aq) in sea water is 0.0537 M. Will Mg(OH)2 precipitate when enough Ca(OH)2 is added to give a [OH–] of 0.0010 M?
Solution
Calculation of the reaction quotient under these conditions is shown here:
Because Q is greater than Ksp (Q = 5.4 \(×\) 10–8 is larger than Ksp = 8.9 \(×\) 10–12), the reverse reaction will proceed, precipitating magnesium hydroxide until the dissolved ion concentrations have been sufficiently lowered, so that Qsp = Ksp.
Check Your Learning Predict whether CaHPO4 will precipitate from a solution with [Ca2+] = 0.0001 M and \(\left[{\text{HPO}}_{4}{}^{\text{2−}}\right]\) = 0.001 M.
No precipitation of CaHPO4; Q = 1 \(×\) 10–7, which is less than Ksp (7 × 10–7)
Precipitation of AgCl Does silver chloride precipitate when equal volumes of a 2.0 \(×\) 10–4-M solution of AgNO3 and a 2.0 \(×\) 10–4-M solution of NaCl are mixed?
Solution The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is:
The solubility product is 1.6 \(×\) 10–10 (see Appendix J).
AgCl will precipitate if the reaction quotient calculated from the concentrations in the mixture of AgNO3 and NaCl is greater than Ksp. Because the volume doubles when equal volumes of AgNO3 and NaCl solutions are mixed, each concentration is reduced to half its initial value
The reaction quotient, Q, is greater than Ksp for AgCl, so a supersaturated solution is formed:
AgCl will precipitate from the mixture until the dissolution equilibrium is established, with Q equal to Ksp.
Check Your Learning Will KClO4 precipitate when 20 mL of a 0.050-M solution of K+ is added to 80 mL of a 0.50-M solution of \({\text{ClO}}_{4}{}^{\text{−}}?\) (Hint: Use the dilution equation to calculate the concentrations of potassium and perchlorate ions in the mixture.)
No, Q = 4.0 \(×\) 10–3, which is less than Ksp = 1.05 \(×\) 10–2
Precipitation of Calcium Oxalate Blood will not clot if calcium ions are removed from its plasma. Some blood collection tubes contain salts of the oxalate ion, \({\text{C}}_{2}{\text{O}}_{4}{}^{\text{2−}},\) for this purpose ((Figure)). At sufficiently high concentrations, the calcium and oxalate ions form solid, CaC2O4·H2O (calcium oxalate monohydrate). The concentration of Ca2+ in a sample of blood serum is 2.2 \(×\) 10–3M. What concentration of \({\text{C}}_{2}{\text{O}}_{4}{}^{\text{2−}}\) ion must be established before CaC2O4·H2O begins to precipitate?
SolutionThe equilibrium expression is:
For this reaction:
(see Appendix J)
Substitute the provided calcium ion concentration into the solubility product expression and solve for oxalate concentration:
A concentration of \(\left[{\text{C}}_{2}{\text{O}}_{4}{}^{\text{2−}}\right]\) = 8.9 \(×\) 10–6M is necessary to initiate the precipitation of CaC2O4 under these conditions.
Check Your Learning If a solution contains 0.0020 mol of \({\text{CrO}}_{4}{}^{\text{2−}}\) per liter, what concentration of Ag+ ion must be reached by adding solid AgNO3 before Ag2CrO4 begins to precipitate? Neglect any increase in volume upon adding the solid silver nitrate.
6.7 \(×\) 10–5M
Concentrations Following Precipitation Clothing washed in water that has a manganese [Mn2+(aq)] concentration exceeding 0.1 mg/L (1.8 \(×\) 10–6M) may be stained by the manganese upon oxidation, but the amount of Mn2+ in the water can be decreased by adding a base to precipitate Mn(OH)2. What pH is required to keep [Mn2+] equal to 1.8 \(×\) 10–6M?
Solution The dissolution of Mn(OH)2 is described by the equation:
At equilibrium:
or
so
Calculate the pH from the pOH:
(final result rounded to one significant digit, limited by the certainty of the Ksp)
Check Your Learning The first step in the preparation of magnesium metal is the precipitation of Mg(OH)2 from sea water by the addition of Ca(OH)2. The concentration of Mg2+(aq) in sea water is 5.37 \(×\) 10–2M. Calculate the pH at which [Mg2+] is decreased to 1.0 \(×\) 10–5M
10.97
In solutions containing two or more ions that may form insoluble compounds with the same counter ion, an experimental strategy called selective precipitation may be used to remove individual ions from solution. By increasing the counter ion concentration in a controlled manner, ions in solution may be precipitated individually, assuming their compound solubilities are adequately different. In solutions with equal concentrations of target ions, the ion forming the least soluble compound will precipitate first (at the lowest concentration of counter ion), with the other ions subsequently precipitating as their compound’s solubilities are reached. As an illustration of this technique, the next example exercise describes separation of a two halide ions via precipitation of one as a silver salt.
Solubility equilibria are useful tools in the treatment of wastewater carried out in facilities that may treat the municipal water in your city or town ((Figure)). Specifically, selective precipitation is used to remove contaminants from wastewater before it is released back into natural bodies of water. For example, phosphate ions \({\text{(PO}}_{4}{}^{\text{3−}}\right)\) are often present in the water discharged from manufacturing facilities. An abundance of phosphate causes excess algae to grow, which impacts the amount of oxygen available for marine life as well as making water unsuitable for human consumption.

One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, Ca(OH)2. As the water is made more basic, the calcium ions react with phosphate ions to produce hydroxylapatite, Ca5(PO4)3OH, which then precipitates out of the solution:
Because the amount of calcium ion added does not result in exceeding the solubility products for other calcium salts, the anions of those salts remain behind in the wastewater. The precipitate is then removed by filtration and the water is brought back to a neutral pH by the addition of CO2 in a recarbonation process. Other chemicals can also be used for the removal of phosphates by precipitation, including iron(III) chloride and aluminum sulfate.
View this site for more information on how phosphorus is removed from wastewater.
Precipitation of Silver Halides A solution contains 0.00010 mol of KBr and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgBr or solid AgCl?
Solution The two equilibria involved are:
If the solution contained about equal concentrations of Cl– and Br–, then the silver salt with the smaller Ksp (AgBr) would precipitate first. The concentrations are not equal, however, so the [Ag+] at which AgCl begins to precipitate and the [Ag+] at which AgBr begins to precipitate must be calculated. The salt that forms at the lower [Ag+] precipitates first.
AgBr precipitates when Q equals Ksp for AgBr
AgI begins to precipitate when [Ag+] is 5.0 \(×\) 10–9M.
For AgCl: AgCl precipitates when Q equals Ksp for AgCl (1.6 \(×\) 10–10). When [Cl–] = 0.10 M:
AgCl begins to precipitate when [Ag+] is 1.6 \(×\) 10–9M.
AgCl begins to precipitate at a lower [Ag+] than AgBr, so AgCl begins to precipitate first. Note the chloride ion concentration of the initial mixture was significantly greater than the bromide ion concentration, and so silver chloride precipitated first despite having a Ksp greater than that of silver bromide.
Check Your Learning If silver nitrate solution is added to a solution which is 0.050 M in both Cl– and Br– ions, at what [Ag+] would precipitation begin, and what would be the formula of the precipitate?
[Ag+] = 1.0 \(×\) 10–11M; AgBr precipitates first
Common Ion Effect
Compared with pure water, the solubility of an ionic compound is less in aqueous solutions containing a common ion (one also produced by dissolution of the ionic compound). This is an example of a phenomenon known as the common ion effect, which is a consequence of the law of mass action that may be explained using Le ChÂtelier’s principle. Consider the dissolution of silver iodide:
This solubility equilibrium may be shifted left by the addition of either silver(I) or iodide ions, resulting in the precipitation of AgI and lowered concentrations of dissolved Ag+ and I–. In solutions that already contain either of these ions, less AgI may be dissolved than in solutions without these ions.
This effect may also be explained in terms of mass action as represented in the solubility product expression:
The mathematical product of silver(I) and iodide ion molarities is constant in an equilibrium mixture regardless of the source of the ions, and so an increase in one ion’s concentration must be balanced by a proportional decrease in the other.
View this simulation to explore various aspects of the common ion effect.
View this simulation to see how the common ion effect works with different concentrations of salts.
Common Ion Effect on Solubility What is the effect on the amount of solid Mg(OH)2 and the concentrations of Mg2+ and OH– when each of the following are added to a saturated solution of Mg(OH)2?
(a) MgCl2
(b) KOH
(c) NaNO3
(d) Mg(OH)2
Solution The solubility equilibrium is
(a) The reaction shifts to the left to relieve the stress produced by the additional Mg2+ ion, in accordance with Le Châtelier’s principle. In quantitative terms, the added Mg2+ causes the reaction quotient to be larger than the solubility product (Q > Ksp), and Mg(OH)2 forms until the reaction quotient again equals Ksp. At the new equilibrium, [OH–] is less and [Mg2+] is greater than in the solution of Mg(OH)2 in pure water. More solid Mg(OH)2 is present.
(b) The reaction shifts to the left to relieve the stress of the additional OH– ion. Mg(OH)2 forms until the reaction quotient again equals Ksp. At the new equilibrium, [OH–] is greater and [Mg2+] is less than in the solution of Mg(OH)2 in pure water. More solid Mg(OH)2 is present.
(c) The concentration of OH– is reduced as the OH– reacts with the acid. The reaction shifts to the right to
(a) Adding a common ion, Mg2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the concentration of hydroxide ion and increasing the amount of undissolved magnesium hydroxide.
(b) Adding a common ion, OH–, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the concentration of magnesium ion and increasing the amount of undissolved magnesium hydroxide.
(c) The added compound does not contain a common ion, and no effect on the magnesium hydroxide solubility equilibrium is expected.
(d) Adding more solid magnesium hydroxide will increase the amount of undissolved compound in the mixture. The solution is already saturated, though, so the concentrations of dissolved magnesium and hydroxide ions will remain the same.
Thus, changing the amount of solid magnesium hydroxide in the mixture has no effect on the value of Q, and no shift is required to restore Q to the value of the equilibrium constant.
Check Your Learning What is the effect on the amount of solid NiCO3 and the concentrations of Ni2+ and \({\text{CO}}_{3}{}^{\text{2−}}\) when each of the following are added to a saturated solution of NiCO3
(a) Ni(NO3)2
(b) KClO4
(c) NiCO3
(d) K2CO3
(a) mass of NiCO3(s) increases, [Ni2+] increases, \(\left[{\text{CO}}_{3}{}^{\text{2−}}\right]\) decreases; (b) no appreciable effect; (c) no effect except to increase the amount of solid NiCO3; (d) mass of NiCO3(s) increases, [Ni2+] decreases, \(\left[{\text{CO}}_{3}{}^{\text{2−}}\right]\) increases;
Common Ion Effect Calculate the molar solubility of cadmium sulfide (CdS) in a 0.010-M solution of cadmium bromide (CdBr2). The Ksp of CdS is 1.0 \(×\) 10–28.
Solution This calculation can be performed using the ICE approach:
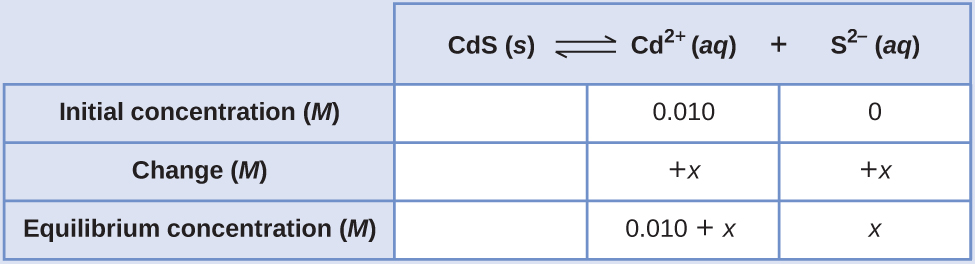
Because Ksp is very small, assume x << 0.010 and solve the simplified equation for x:
The molar solubility of CdS in this solution is 1.0 \(×\) 10–26M.
Check Your Learning Calculate the molar solubility of aluminum hydroxide, Al(OH)3, in a 0.015-M solution of aluminum nitrate, Al(NO3)3. The Ksp of Al(OH)3 is 2 \(×\) 10–32.
4 \(×\) 10–11M
Key Concepts and Summary
The equilibrium constant for an equilibrium involving the precipitation or dissolution of a slightly soluble ionic solid is called the solubility product, Ksp, of the solid. For a heterogeneous equilibrium involving the slightly soluble solid MpXq and its ions Mm+ and Xn–:
the solubility product expression is:
The solubility product of a slightly soluble electrolyte can be calculated from its solubility; conversely, its solubility can be calculated from its Ksp, provided the only significant reaction that occurs when the solid dissolves is the formation of its ions.
A slightly soluble electrolyte begins to precipitate when the magnitude of the reaction quotient for the dissolution reaction exceeds the magnitude of the solubility product. Precipitation continues until the reaction quotient equals the solubility product.
Key Equations
- \({\text{M}}_{p}{\text{X}}_{q}\left(s\right)⇌p{\text{M}}^{\text{m+}}\left(aq\right)+q{\text{X}}^{\text{n−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}={\left[\text{M}}^{\text{m+}}{\right]}^{p}{{\left[\text{X}}^{\text{n−}}\right]}^{q}\)
Chemistry End of Chapter Exercises
Complete the changes in concentrations for each of the following reactions:
(a) \(\begin{array}{ccc}\text{AgI}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Ag}}^{\text{+}}\left(aq\right)& +\phantom{\rule{0.2em}{0ex}}{\text{I}}^{\text{−}}\left(aq\right)\\ \\ & x& _____\end{array}\)
(b) \(\begin{array}{ccc}{\text{CaCO}}_{3}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Ca}}^{\text{2+}}\left(aq\right)+& {\text{CO}}_{3}{}^{\text{2−}}\left(aq\right)\\ \\ & ____& x\end{array}\)
(c) \(\begin{array}{ccc}\text{Mg}{\left(\text{OH}\right)}_{2}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Mg}}^{\text{2+}}\left(aq\right)+& 2{\text{OH}}^{\text{−}}\left(aq\right)\\ \\ & x& _____\end{array}\)
(d) \(\begin{array}{ccc}{\text{Mg}}_{3}\left({\text{PO}}_{4}{\right)}_{2}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& 3{\text{Mg}}^{\text{2+}}\left(aq\right)+& 2{\text{PO}}_{4}{}^{\text{3−}}\left(aq\right)\\ \\ & & x_____\end{array}\)
(e) \(\begin{array}{cccc}{\text{Ca}}_{5}\left({\text{PO}}_{4}{\right)}_{3}\text{OH}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& 5{\text{Ca}}^{\text{2+}}\left(aq\right)+& 3{\text{PO}}_{4}{}^{\text{3−}}\left(aq\right)+& {\text{OH}}^{\text{−}}\left(aq\right)\\ \\ & _____& _____& x\end{array}\)
(a) \(\begin{array}{ccc}\text{AgI}\left(s\right)⇌& {\text{Ag}}^{\text{+}}\left(aq\right)+& {\text{I}}^{\text{−}}\left(aq\right)\\ \\ & x& \underset{_}{x}\end{array}\)
(b) \(\begin{array}{ccc}{\text{CaCO}}_{3}\left(s\right)⇌& {\text{Ca}}^{\text{2+}}\left(aq\right)+& {\text{CO}}_{3}{}^{\text{2−}}\left(aq\right)\\ \\ & \underset{_}{x}& x\end{array}\)
(c) \(\begin{array}{lll}\text{Mg}{\left(\text{OH}\right)}_{2}\left(s\right)⇌\hfill & {\text{Mg}}^{\text{2+}}\left(aq\right)\hfill & +\phantom{\rule{0.2em}{0ex}}2{\text{OH}}^{\text{−}}\left(aq\right)\hfill \\ \hfill & x\hfill & \underset{_}{\text{2}x}\hfill \end{array}\)
(d) \(\begin{array}{ccc}{\text{Mg}}_{3}{\left({\text{PO}}_{4}\right)}_{2}\left(s\right)⇌& {\text{3Mg}}^{\text{2+}}\left(aq\right)+& {\text{2PO}}_{4}{}^{\text{3−}}\left(aq\right)\\ \\ & \underset{_}{3x}& 2x\end{array}\)
(e) \(\begin{array}{cccc}{\text{Ca}}_{5}\left({\text{PO}}_{4}{\right)}_{3}\text{OH}\left(s\right)⇌& {\text{5Ca}}^{\text{2+}}\left(aq\right)+& {\text{3PO}}_{4}{}^{\text{3−}}\left(aq\right)+& {\text{OH}}^{\text{−}}\left(aq\right)\\ \\ & \underset{_}{5x}& \underset{_}{3x}& x\end{array}\)
Complete the changes in concentrations for each of the following reactions:
(a) \(\begin{array}{ccc}{\text{BaSO}}_{4}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Ba}}^{\text{2+}}\left(aq\right)+& {\text{SO}}_{4}{}^{\text{2−}}\left(aq\right)\\ \\ & x& _____\end{array}\)
(b) \(\begin{array}{ccc}{\text{Ag}}_{2}{\text{SO}}_{4}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& 2{\text{Ag}}^{\text{+}}\left(aq\right)+& {\text{SO}}_{4}{}^{\text{2−}}\left(aq\right)\\ \\ & _____& x\end{array}\)
(c) \(\begin{array}{ccc}\text{Al}{\left(\text{OH}\right)}_{3}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Al}}^{\text{3+}}\left(aq\right)+& 3{\text{OH}}^{\text{−}}\left(aq\right)\\ \\ & x& _____\end{array}\)
(d) \(\begin{array}{cccc}\text{Pb}\left(\text{OH}\right)\text{Cl}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Pb}}^{\text{2+}}\left(aq\right)+& {\text{OH}}^{\text{−}}\left(aq\right)+& {\text{Cl}}^{\text{−}}\left(aq\right)\\ \\ & _____\phantom{\rule{0.2em}{0ex}}& x& _____\end{array}\)
(e) \(\begin{array}{ccc}{\text{Ca}}_{3}\left({\text{AsO}}_{4}{\right)}_{2}\left(s\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& 3{\text{Ca}}^{\text{2+}}\left(aq\right)+& 2{\text{AsO}}_{4}{}^{\text{3−}}\left(aq\right)\\ \\ & 3x& _____\end{array}\)
How do the concentrations of Ag+ and \({\text{CrO}}_{4}{}^{\text{2−}}\) in a saturated solution above 1.0 g of solid Ag2CrO4 change when 100 g of solid Ag2CrO4 is added to the system? Explain.
There is no change. A solid has an activity of 1 whether there is a little or a lot.
How do the concentrations of Pb2+ and S2– change when K2S is added to a saturated solution of PbS?
What additional information do we need to answer the following question: How is the equilibrium of solid silver bromide with a saturated solution of its ions affected when the temperature is raised?
The solubility of silver bromide at the new temperature must be known. Normally the solubility increases and some of the solid silver bromide will dissolve.
Which of the following slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: CoSO3, CuI, PbCO3, PbCl2, Tl2S, KClO4?
Which of the following slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: AgCl, BaSO4, CaF2, Hg2I2, MnCO3, ZnS, PbS?
CaF2, MnCO3, and ZnS
Write the ionic equation for dissolution and the solubility product (Ksp) expression for each of the following slightly soluble ionic compounds:
(a) PbCl2
(b) Ag2S
(c) Sr3(PO4)2
(d) SrSO4
Write the ionic equation for the dissolution and the Ksp expression for each of the following slightly soluble ionic compounds:
(a) LaF3
(b) CaCO3
(c) Ag2SO4
(d) Pb(OH)2
(a) \({\text{LaF}}_{3}\left(s\right)⇌{\text{La}}^{\text{3+}}\left(aq\right)+{\text{3F}}^{\text{−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=\left[{\text{La}}^{\text{3+}}\right]{\left[\text{F}}^{\text{−}}{\right]}^{3};\)
(b) \({\text{CaCO}}_{3}\left(s\right)⇌{\text{Ca}}^{\text{2+}}\left(aq\right)+{\text{CO}}_{3}{}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=\left[{\text{Ca}}^{\text{2+}}\right]\left[{\text{CO}}_{3}{}^{\text{2−}}\right];\)
(c) \({\text{Ag}}_{2}{\text{SO}}_{4}\left(s\right)⇌{\text{2Ag}}^{\text{+}}\left(aq\right)+{\text{SO}}_{4}{}^{\text{2−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}={\left[{\text{Ag}}^{\text{+}}\right]}^{2}\left[{\text{SO}}_{4}{}^{\text{2−}}\right];\)
(d) \({\text{Pb(OH)}}_{2}\left(s\right)⇌{\text{Pb}}^{\text{2+}}\left(aq\right)+{\text{2OH}}^{\text{−}}\left(aq\right)\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=\left[{\text{Pb}}^{\text{2+}}\right]{\left[{\text{OH}}^{\text{−}}\right]}^{2}\)
The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.
(a) BaSiF6, 0.026 g/100 mL (contains \({\text{SiF}}_{6}{}^{\text{2−}}\) ions)
(b) Ce(IO3)4, 1.5 \(×\) 10–2 g/100 mL
(c) Gd2(SO4)3, 3.98 g/100 mL
(d) (NH4)2PtBr6, 0.59 g/100 mL (contains \({\text{PtBr}}_{6}{}^{\text{2−}}\) ions)
The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.
(a) BaSeO4, 0.0118 g/100 mL
(b) Ba(BrO3)2·H2O, 0.30 g/100 mL
(c) NH4MgAsO4·6H2O, 0.038 g/100 mL
(d) La2(MoO4)3, 0.00179 g/100 mL
(a)1.77 \(×\) 10–7; (b) 1.6 \(×\) 10–6; (c) 2.2 \(×\) 10–9; (d) 7.91 \(×\) 10–22
Use solubility products and predict which of the following salts is the most soluble, in terms of moles per liter, in pure water: CaF2, Hg2Cl2, PbI2, or Sn(OH)2.
Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product:
(a) KHC4H4O6
(b) PbI2
(c) Ag4[Fe(CN)6], a salt containing the \({\text{Fe(CN)}}_{4}{}^{\text{−}}\) ion
(d) Hg2I2
(a) 2 \(×\) 10–2M; (b) 1.5 \(×\) 10–3M; (c) 2.27 \(×\) 10–9M; (d) 2.2 \(×\) 10–10M
Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product:
(a) Ag2SO4
(b) PbBr2
(c) AgI
(d) CaC2O4·H2O
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected.
(a) AgCl(s) in 0.025 M NaCl
(b) CaF2(s) in 0.00133 M KF
(c) Ag2SO4(s) in 0.500 L of a solution containing 19.50 g of K2SO4
(d) Zn(OH)2(s) in a solution buffered at a pH of 11.45
(a) 6.4 \(×\) 10−9M = [Ag+], [Cl−] = 0.025 M. Check: \(\frac{6.4\phantom{\rule{0.3em}{0ex}}×\phantom{\rule{0.3em}{0ex}}{10}^{-9}\phantom{\rule{0.2em}{0ex}}M}{0.025\phantom{\rule{0.2em}{0ex}}M}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}\text{100%}=\text{2.6}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5}%,\)an insignificant change;
(b) 2.2 \(×\) 10−5M = [Ca2+], [F−] = 0.0013 M. Check: \(\frac{\text{2.26}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5}\phantom{\rule{0.2em}{0ex}}M}{0.00133\phantom{\rule{0.2em}{0ex}}M}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}\text{100%}=\text{1.70%}.\) This value is less than 5% and can be ignored.
(c) 0.2238 M = \(\left[{\text{SO}}_{4}{}^{2\text{−}}\right];\) [Ag+] = 7.4 \(×\) 10–3M. Check: \(\frac{3.7\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}}{0.2238}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100%=\text{1.64}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-2};\) the condition is satisfied.
(d) [OH–] = 2.8 \(×\) 10–3M; 5.7 \(×\) 10−12M = [Zn2+]. Check: \(\frac{5.7\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-12}}{2.8\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}\text{100%}=\text{2.0}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-7}%;\) x is less than 5% of [OH–] and is, therefore, negligible.
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected.
(a) TlCl(s) in 1.250 M HCl
(b) PbI2(s) in 0.0355 M CaI2
(c) Ag2CrO4(s) in 0.225 L of a solution containing 0.856 g of K2CrO4
(d) Cd(OH)2(s) in a solution buffered at a pH of 10.995
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that it is not appropriate to neglect the changes in the initial concentrations of the common ions.
(a) TlCl(s) in 0.025 M TlNO3
(b) BaF2(s) in 0.0313 M KF
(c) MgC2O4 in 2.250 L of a solution containing 8.156 g of Mg(NO3)2
(d) Ca(OH)2(s) in an unbuffered solution initially with a pH of 12.700
(a) [Cl–] = 7.6 \(×\) 10−3M
Check: \(\frac{7.6\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}}{0.025}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100%=30%\)
This value is too large to drop x. Therefore solve by using the quadratic equation:
[Ti+] = 3.1 \(×\) 10–2M
[Cl–] = 6.1 \(×\) 10–3
(b) [Ba2+] = 7.7 \(×\) 10–4M
Check: \(\frac{7.7\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-4}}{0.0313}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100%=2.4%\)
Therefore, the condition is satisfied.
[Ba2+] = 7.7 \(×\) 10–4M
[F–] = 0.0321 M;
(c) Mg(NO3)2 = 0.02444 M
\(\left[{\text{C}}_{2}{\text{O}}_{4}{}^{\text{2−}}\right]=2.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−5}}\)
Check: \(\frac{2.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−5}}}{0.02444}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100%=0.12%\)
The condition is satisfied; the above value is less than 5%.
\(\left[{\text{C}}_{2}{\text{O}}_{4}{}^{\text{2−}}\right]=2.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−5}}\phantom{\rule{0.2em}{0ex}}M\)
[Mg2+] = 0.0244 M
(d) [OH–] = 0.0501 M
[Ca2+] = 3.15 \(×\) 10–3
Check: \(\frac{3.15\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{\text{−3}}}{0.050}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100%=6.28%\)
This value is greater than 5%, so a more exact method, such as successive approximations, must be used.
[Ca2+] = 2.8 \(×\) 10–3M
[OH–] = 0.053 \(×\) 10–2M
Explain why the changes in concentrations of the common ions in (Figure) can be neglected.
Explain why the changes in concentrations of the common ions in (Figure) cannot be neglected.
The changes in concentration are greater than 5% and thus exceed the maximum value for disregarding the change.
Calculate the solubility of aluminum hydroxide, Al(OH)3, in a solution buffered at pH 11.00.
Refer to Appendix J for solubility products for calcium salts. Determine which of the calcium salts listed is most soluble in moles per liter and which is most soluble in grams per liter.
CaSO4∙2H2O is the most soluble Ca salt in mol/L, and it is also the most soluble Ca salt in g/L.
Most barium compounds are very poisonous; however, barium sulfate is often administered internally as an aid in the X-ray examination of the lower intestinal tract ((Figure)). This use of BaSO4 is possible because of its low solubility. Calculate the molar solubility of BaSO4 and the mass of barium present in 1.00 L of water saturated with BaSO4.
Public Health Service standards for drinking water set a maximum of 250 mg/L (2.60 \(×\) 10–3M) of \({\text{SO}}_{4}{}^{\text{2−}}\) because of its cathartic action (it is a laxative). Does natural water that is saturated with CaSO4 (“gyp” water) as a result or passing through soil containing gypsum, CaSO4·2H2O, meet these standards? What is the concentration of \({\text{SO}}_{4}{}^{\text{2−}}\) in such water?
4.8 \(×\) 10–3M = \(\left[{\text{SO}}_{4}{}^{\text{2−}}\right]\) = [Ca2+]; Since this concentration is higher than 2.60 \(×\) 10–3M, “gyp” water does not meet the standards.
Perform the following calculations:
(a) Calculate [Ag+] in a saturated aqueous solution of AgBr.
(b) What will [Ag+] be when enough KBr has been added to make [Br–] = 0.050 M?
(c) What will [Br–] be when enough AgNO3 has been added to make [Ag+] = 0.020 M?
The solubility product of CaSO4·2H2O is 2.4 \(×\) 10–5. What mass of this salt will dissolve in 1.0 L of 0.010 M \({\text{SO}4}_{}{}^{\text{2−}}?\)
Mass (CaSO4·2H2O) = 0.72 g/L
Assuming that no equilibria other than dissolution are involved, calculate the concentrations of ions in a saturated solution of each of the following (see Appendix J for solubility products).
(a) TlCl
(b) BaF2
(c) Ag2CrO4
(d) CaC2O4·H2O
(e) the mineral anglesite, PbSO4
Assuming that no equilibria other than dissolution are involved, calculate the concentrations of ions in a saturated solution of each of the following (see Appendix J for solubility products):
(a) AgI
(b) Ag2SO4
(c) Mn(OH)2
(d) Sr(OH)2·8H2O
(e) the mineral brucite, Mg(OH)2
(a) [Ag+] = [I–] = 1.3 \(×\) 10–5M; (b) [Ag+] = 2.88 \(×\) 10–2M, \(\left[{\text{SO}}_{4}{}^{\text{2−}}\right]\)= 1.44 \(×\) 10–2M; (c) [Mn2+] = 3.7 \(×\) 10–5M, [OH–] = 7.4 \(×\) 10–5M; (d) [Sr2+] = 4.3 \(×\) 10–2M, [OH–] = 8.6 \(×\) 10–2M; (e) [Mg2+] = 1.3 \(×\) 10–4M, [OH–] = 2.6 \(×\) 10–4M.
The following concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate Ksp for each of the slightly soluble solids indicated:
(a) AgBr: [Ag+] = 5.7 \(×\) 10–7M, [Br–] = 5.7 \(×\) 10–7M
(b) CaCO3: [Ca2+] = 5.3 \(×\) 10–3M, \(\left[{\text{CO}}_{3}{}^{\text{2−}}\right]\) = 9.0 \(×\) 10–7M
(c) PbF2: [Pb2+] = 2.1 \(×\) 10–3M, [F–] = 4.2 \(×\) 10–3M
(d) Ag2CrO4: [Ag+] = 5.3 \(×\) 10–5M, 3.2 \(×\) 10–3M
(e) InF3: [In3+] = 2.3 \(×\) 10–3M, [F–] = 7.0 \(×\) 10–3M
The following concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate Ksp for each of the slightly soluble solids indicated:
(a) TlCl: [Tl+] = 1.21 \(×\) 10–2M, [Cl–] = 1.2 \(×\) 10–2M
(b) Ce(IO3)4: [Ce4+] = 1.8 \(×\) 10–4M, \(\left[{\text{IO}}_{3}{}^{\text{−}}\right]\) = 2.6 \(×\) 10–13M
(c) Gd2(SO4)3: [Gd3+] = 0.132 M, \(\left[{\text{SO}}_{\text{4}}{}^{\text{2−}}\right]\) = 0.198 M
(d) Ag2SO4: [Ag+] = 2.40 \(×\) 10–2M, \(\left[{\text{SO}}_{\text{4}}{}^{\text{2−}}\right]\) = 2.05 \(×\) 10–2M
(e) BaSO4: [Ba2+] = 0.500 M, \(\left[{\text{SO}}_{\text{4}}{}^{\text{2−}}\right]\) = 4.6 \(×\) 10−8M
(a) 1.7 \(×\) 10–4; (b) 8.2 \(×\) 10–55; (c) 1.35 \(×\) 10–4; (d) 1.18 \(×\) 10–5; (e) 1.08 \(×\) 10–10
Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Appendix J for Ksp values.)
(a) KClO4: [K+] = 0.01 M, \(\left[{\text{ClO}}_{4}{}^{\text{−}}\right]\) = 0.01 M
(b) K2PtCl6: [K+] = 0.01 M, \(\left[{\text{PtCl}}_{\text{6}}{}^{\text{2−}}\right]\) = 0.01 M
(c) PbI2: [Pb2+] = 0.003 M, [I–] = 1.3 \(×\) 10–3M
(d) Ag2S: [Ag+] = 1 \(×\) 10–10M, [S2–] = 1 \(×\) 10–13M
Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Appendix J for Ksp values.)
(a) CaCO3: [Ca2+] = 0.003 M, \(\left[{\text{CO}}_{\text{3}}{}^{\text{2−}}\right]\) = 0.003 M
(b) Co(OH)2: [Co2+] = 0.01 M, [OH–] = 1 \(×\) 10–7M
(c) CaHPO4: [Ca2+] = 0.01 M, \(\left[{\text{HPO}}_{\text{4}}{}^{\text{2−}}\right]\) = 2 \(×\) 10–6M
(d) Pb3(PO4)2: [Pb2+] = 0.01 M, \(\left[{\text{PO}}_{\text{4}}{}^{\text{3−}}\right]\)= 1 \(×\) 10–13M
(a) CaCO3 does precipitate. (b) The compound does not precipitate. (c) The compound does not precipitate. (d) The compound precipitates.
Calculate the concentration of Tl+ when TlCl just begins to precipitate from a solution that is 0.0250 M in Cl–.
Calculate the concentration of sulfate ion when BaSO4 just begins to precipitate from a solution that is 0.0758 M in Ba2+.
3.03 \(×\) 10−7M
Calculate the concentration of Sr2+ when SrF2 starts to precipitate from a solution that is 0.0025 M in F–.
Calculate the concentration of \({\text{PO}}_{4}{}^{\text{3−}}\) when Ag3PO4 starts to precipitate from a solution that is 0.0125 M in Ag+.
9.2 \(×\) 10−13M
Calculate the concentration of F– required to begin precipitation of CaF2 in a solution that is 0.010 M in Ca2+.
Calculate the concentration of Ag+ required to begin precipitation of Ag2CO3 in a solution that is 2.50 \(×\) 10–6M in \({\text{CO}}_{3}{}^{\text{2−}}.\)
[Ag+] = 1.8 \(×\) 10–3M
What [Ag+] is required to reduce \(\left[{\text{CO}}_{3}{}^{\text{2−}}\right]\) to 8.2 \(×\) 10–4M by precipitation of Ag2CO3?
What [F–] is required to reduce [Ca2+] to 1.0 \(×\) 10–4M by precipitation of CaF2?
6.3 \(×\) 10–4
A volume of 0.800 L of a 2 \(×\) 10–4-M Ba(NO3)2 solution is added to 0.200 L of 5 \(×\) 10–4M Li2SO4. Does BaSO4 precipitate? Explain your answer.
Perform these calculations for nickel(II) carbonate. (a) With what volume of water must a precipitate containing NiCO3 be washed to dissolve 0.100 g of this compound? Assume that the wash water becomes saturated with NiCO3 (Ksp = 1.36 \(×\) 10–7).
(b) If the NiCO3 were a contaminant in a sample of CoCO3 (Ksp = 1.0 \(×\) 10–12), what mass of CoCO3 would have been lost? Keep in mind that both NiCO3 and CoCO3 dissolve in the same solution.
(a) 2.25 L; (b) 7.2 \(×\) 10–7 g
Iron concentrations greater than 5.4 \(×\) 10–6M in water used for laundry purposes can cause staining. What [OH–] is required to reduce [Fe2+] to this level by precipitation of Fe(OH)2?
A solution is 0.010 M in both Cu2+ and Cd2+. What percentage of Cd2+ remains in the solution when 99.9% of the Cu2+ has been precipitated as CuS by adding sulfide?
100% of it is dissolved
A solution is 0.15 M in both Pb2+ and Ag+. If Cl– is added to this solution, what is [Ag+] when PbCl2 begins to precipitate?
What reagent might be used to separate the ions in each of the following mixtures, which are 0.1 M with respect to each ion? In some cases it may be necessary to control the pH. (Hint: Consider the Ksp values given in Appendix J.)
(a) \({\text{Hg}}_{2}{}^{\text{2+}}\) and Cu2+
(b) \({\text{SO}}_{4}{}^{\text{2−}}\) and Cl–
(c) Hg2+ and Co2+
(d) Zn2+ and Sr2+
(e) Ba2+ and Mg2+
(f) \({\text{CO}}_{3}{}^{\text{2−}}\) and OH–
(a) \({\text{Hg}}_{2}{}^{\text{2+}}\) and Cu2+: Add \({\text{SO}}_{4}{}^{\text{2−}}.\) (b) \({\text{SO}}_{4}{}^{\text{2−}}\) and Cl–: Add Ba2+. (c) Hg2+ and Co2+: Add S2–. (d) Zn2+ and Sr2+: Add OH– until [OH–] = 0.050 M. (e) Ba2+ and Mg2+: Add \({\text{SO}}_{4}{}^{\text{2−}}.\) (f) \({\text{CO}}_{3}{}^{\text{2−}}\) and OH–: Add Ba2+.
A solution contains 1.0 \(×\) 10–5 mol of KBr and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgBr or solid AgCl?
A solution contains 1.0 \(×\) 10–2 mol of KI and 0.10 mol of KCl per liter. AgNO3 is gradually added to this solution. Which forms first, solid AgI or solid AgCl?
AgI will precipitate first.
The calcium ions in human blood serum are necessary for coagulation ((Figure)). Potassium oxalate, K2C2O4, is used as an anticoagulant when a blood sample is drawn for laboratory tests because it removes the calcium as a precipitate of CaC2O4·H2O. It is necessary to remove all but 1.0% of the Ca2+ in serum in order to prevent coagulation. If normal blood serum with a buffered pH of 7.40 contains 9.5 mg of Ca2+ per 100 mL of serum, what mass of K2C2O4 is required to prevent the coagulation of a 10 mL blood sample that is 55% serum by volume? (All volumes are accurate to two significant figures. Note that the volume of serum in a 10-mL blood sample is 5.5 mL. Assume that the Ksp value for CaC2O4 in serum is the same as in water.)
About 50% of urinary calculi (kidney stones) consist of calcium phosphate, Ca3(PO4)2. The normal mid range calcium content excreted in the urine is 0.10 g of Ca2+ per day. The normal mid range amount of urine passed may be taken as 1.4 L per day. What is the maximum concentration of phosphate ion that urine can contain before a calculus begins to form?
1.5 \(×\) 10−12M
The pH of normal urine is 6.30, and the total phosphate concentration \(\left({\left[\text{PO}}_{4}{}^{\text{3−}}\right]\) + \(\left[{\text{HPO}}_{4}{}^{\text{2−}}\right]\) + \(\left[{\text{H}}_{2}{\text{PO}}_{4}{}^{\text{−}}\right]\) + [H3PO4]) is 0.020 M. What is the minimum concentration of Ca2+ necessary to induce kidney stone formation? (See (Figure) for additional information.)
Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions:
\({\text{Mg}}^{\text{2+}}\left(aq\right)+{\text{Ca(OH)}}_{2}\left(aq\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{\text{Mg(OH)}}_{2}\left(s\right)+{\text{Ca}}^{\text{2+}}\left(aq\right)\)
\({\text{Mg(OH)}}_{2}\left(s\right)+\text{2HCl(}aq\right)\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{\text{MgCl}}_{2}\left(s\right)+{\text{2H}}_{2}\text{O}\left(l\right)\)
\({\text{MgCl}}_{2}\left(l\right)\stackrel{\phantom{\rule{0.7em}{0ex}}\text{electrolysis}\phantom{\rule{0.7em}{0ex}}}{\to }\text{Mg}\left(s\right)+{\text{Cl}}_{2}\left(g\right)\)
Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium as Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2 is required to precipitate 99.9% of the magnesium in 1.00 \(×\) 103 L of sea water?
3.99 kg
Hydrogen sulfide is bubbled into a solution that is 0.10 M in both Pb2+ and Fe2+ and 0.30 M in HCl. After the solution has come to equilibrium it is saturated with H2S ([H2S] = 0.10 M). What concentrations of Pb2+ and Fe2+ remain in the solution? For a saturated solution of H2S we can use the equilibrium:
(Hint: The \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) changes as metal sulfides precipitate.)
Perform the following calculations involving concentrations of iodate ions:
(a) The iodate ion concentration of a saturated solution of La(IO3)3 was found to be 3.1 \(×\) 10–3 mol/L. Find the Ksp.
(b) Find the concentration of iodate ions in a saturated solution of Cu(IO3)2 (Ksp = 7.4 \(×\) 10–8).
(a) 3.1 \(×\) 10–11; (b) [Cu2+] = 2.6 \(×\) 10–3; \(\left[{\text{IO}}_{3}{}^{\text{−}}\right]\) = 5.3 \(×\) 10–3
Calculate the molar solubility of AgBr in 0.035 M NaBr (Ksp = 5 \(×\) 10–13).
How many grams of Pb(OH)2 will dissolve in 500 mL of a 0.050-M PbCl2 solution (Ksp = 1.2 \(×\) 10–15)?
1.8 \(×\) 10–5 g Pb(OH)2
Use the simulation from the earlier Link to Learning to complete the following exercise. Using 0.01 g CaF2, give the Ksp values found in a 0.2-M solution of each of the salts. Discuss why the values change as you change soluble salts.
How many grams of Milk of Magnesia, Mg(OH)2 (s) (58.3 g/mol), would be soluble in 200 mL of water. Ksp = 7.1 \(×\) 10–12. Include the ionic reaction and the expression for Ksp in your answer. (Kw = 1 \(×\) 10–14 = [H3O+][OH–])
\({\text{Mg(OH)}}_{2}\left(s\right)⇌{\text{Mg}}^{\text{2+}}+{\text{2OH}}^{\text{−}}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=\left[{\text{Mg}}^{\text{2+}}\right]{\left[{\text{OH}}^{\text{−}}\right]}^{2}\)
1.23 \(×\) 10−3 g Mg(OH)2
Two hypothetical salts, LM2 and LQ, have the same molar solubility in H2O. If Ksp for LM2 is 3.20 \(×\) 10–5, what is the Ksp value for LQ?
The carbonate ion concentration is gradually increased in a solution containing divalent cations of magnesium, calcium, strontium, barium, and manganese. Which of the following carbonates will form first? Which of the following will form last? Explain.
(a) \({\text{MgCO}}_{3}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=3.5\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}1{0}^{-8}\)
(b) \({\text{CaCO}}_{3}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=4.2\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}1{0}^{-7}\)
(c) \({\text{SrCO}}_{3}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=3.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}1{0}^{-9}\)
(d) \({\text{BaCO}}_{3}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=4.4\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}1{0}^{-5}\)
(e) \({\text{MnCO}}_{3}\phantom{\rule{4em}{0ex}}{K}_{\text{sp}}=5.1\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}1{0}^{-9}\)
MnCO3 will form first, since it has the smallest Ksp value it is the least soluble. MnCO3 will be the last to precipitate, it has the largest Ksp value.
How many grams of Zn(CN)2(s) (117.44 g/mol) would be soluble in 100 mL of H2O? Include the balanced reaction and the expression for Ksp in your answer. The Ksp value for Zn(CN)2(s) is 3.0 \(×\) 10–16.
Glossary
- common ion effect
- effect on equilibrium when a substance with an ion in common with the dissolved species is added to the solution; causes a decrease in the solubility of an ionic species, or a decrease in the ionization of a weak acid or base
- molar solubility
- solubility of a compound expressed in units of moles per liter (mol/L)
- selective precipitation
- process in which ions are separated using differences in their solubility with a given precipitating reagent
- solubility product constant (Ksp)
- equilibrium constant for the dissolution of an ionic compound

