174 Gene Therapy for Sickle Cell Disease 2018
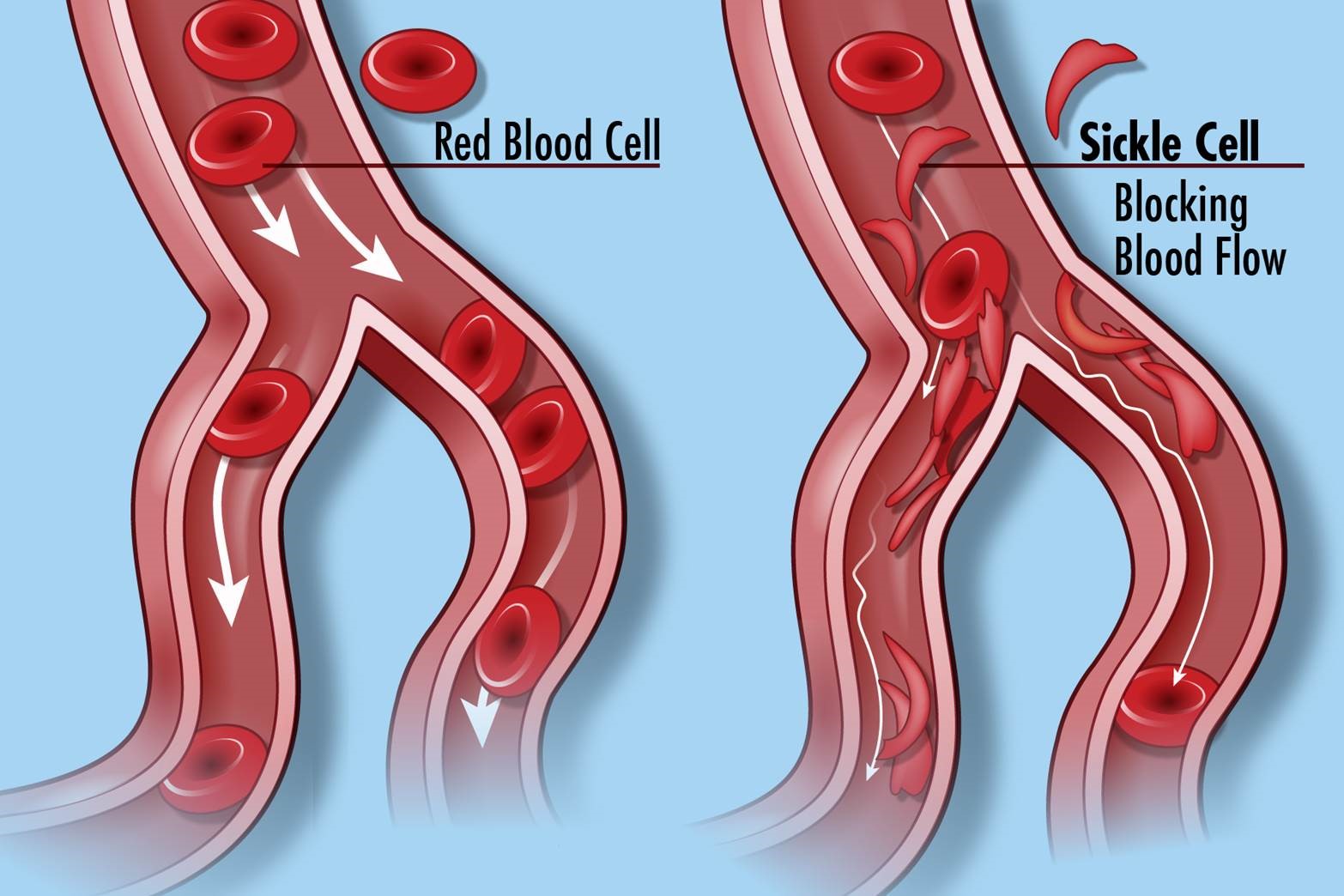
Image credit: “Sickle Cell Disease” by National Institutes of Health (NIH) is licensed under CC BY-NC 2.0.
In sickle cell anemia, a change of one amino acid in hemoglobin molecules distorts the red blood cells and causes them to assume a crescent or “sickle” shape, which clogs blood vessels. Sickle cell anemia can lead to myriad serious health problems such as breathlessness, dizziness, headaches, and abdominal pain for those affected. A change in nucleotide sequence of the gene’s coding region for the hemoglobin βchain causes the disease. (Adapted with modification from chapter 3, Biology 2e, OpenStax). This change or mutation in a single gene allows the possibility for gene therapy.
In 2019, radio stations and newspapers reported about the experimental gene therapy of sickle cell disease patient Victoria Gray. Mrs. Victoria Gray lives in Mississippi with her husband and children. She has suffered from sickle cell disease since early childhood. It has also negatively impacted her college career and the activities she can do with her children. Mrs. Gray was offered participation in an gene therapy experiment using a new technique called CRISPR. In 2019, Mrs. Gray was the only person in the US to receive this type of gene therapy treatment. For this treatment, bone marrow cells were removed from Mrs. Gray and then genetically altered using the new CRISPR technique. The genetically changed cells have a version of hemoglobin gene that will make better-performing hemoglobin. The modified cells are then re-injected into the body of the patient. The treatment is lengthy and did also involve very unpleasant chemotherapy. The treatment showed improvement in quality of life for Mrs. Gray after some months. However, it is not clear if the improvements will last and how long. As the technique is also completely new, there are chances for unforeseen dangerous complications. A scientific study about the 15 months after treatment was published. Three severe adverse events were reported that were treated successfully. Also, 111 less severe events were recorded.
Ethics questions
- Should a scientist or a doctor offer a new experimental treatment that hasn’t been tried on any human being before?
- Should a scientist or doctor include additional ethical considerations if an experimental treatment is offered to a demographic group that is exposed to disproportionally high health disparities?
- Should a scientist or doctor offer a new experimental treatment to a person that is parenting dependent children?
- Is this treatment technique a safe technique?
- Is there value in giving a potentially unsafe treatment to a volunteer with a life-shortening disease?
- Is there a value in testing new treatments for diseases that affect underserved population groups with health disparities?
- Who should be involved in discussions, planning, and decisions about research trying to overcome health disparities?
Further reading
A Young Mississippi Woman’s Journey Through A Pioneering Gene-Editing Experiment. Rob Stein. December 25, 2019. National Public Radio (https://www.npr.org/sections/health-shots/2019/12/25/784395525/a-young-mississippi-womans-journey-through-a-pioneering-gene-editing-experiment):
Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, Foell J, de la Fuente J, Grupp S, Handgretinger R, Ho TW, Kattamis A, Kernytsky A, Lekstrom-Himes J, Li AM, Locatelli F, Mapara MY, de Montalembert M, Rondelli D, Sharma A, Sheth S, Soni S, Steinberg MH, Wall D, Yen A, Corbacioglu S. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med. 2021 Jan 21;384(3):252-260. doi: 10.1056/NEJMoa2031054. Epub 2020 Dec 5. PMID: 33283989. https://www.nejm.org/doi/pdf/10.1056/NEJMoa2031054?articleTools=true accessed 4/6/2022; article is freely available as fulltext download;

