167 History of Ethics in STEM
During the history of science, technology, engineering, and mathematics (STEM), ethical questions or problems arose. Only very few examples are given, and chances are high that there are more important or instructive ones. The later sections list some additional examples in more depth.
Galileo Galilei (1564–1642) and Heliocentrism:
The Italian scientist Galileo proposed a model of planets, the sun, and other bodies with the sun at its center and planets of the solar system circling around it. This clashed with existing models proposing the planet Earth at the center and everything else moving around Earth. It clashed also with religious authorities and beliefs. It is conceivable that Galileo had ethical questions and problems because of his contemporary situation. He was jailed, condemned, and forced to deny his own research findings.[1]
- Had Galileo Galilei a conflict between his scientific views and his religious beliefs?
- Was it ethical for Galileo Galilei to disregard the warnings of the religious authorities at his time and to continue to pursue the scientific research and scientific dissemination of his research?
- Was it ethical for the religious authorities to prosecute Galileo Galilei and condemn him for his research and public dissemination of his findings?


Charles Darwin (1809–1882) and the Origin of Species:
Charles Darwin proposed to his contemporaries that animal species evolved and were not created. His explanations for biological diversity and species raised concerns with prevailing religious views of the origins of animals and biological diversity arising from a creator. Charles Darwin wrote also a second book, entitled The Descent of Man, and Selection in Relation to Sex, wherein he proposes that morality and ethics in humans developed or evolved for biological reasons. It was interpreted as a biologicalization of ethics. However, that proposal of evolutionary ethics has received wide criticism even into contemporary times.[2]
Some of the following concerns were raised:
- Are there dangers if the concept of the survival of a “tribe” or “race” is used as a measuring stick to defend ethical standards in a society?
- Is a nation’s war culture justified for ethical reasons if it allows the nation to survive better against other competing nations?
- Is it ethical to justify cannibalism if a society evolved where cannibalism is practiced?
Rosalind Franklin (1920–1958) and the DNA structure
In 1951, Rosalind Franklin was in charge of setting up a lab for a highly complicated, very advanced technology. She was building a lab for X-ray diffraction or crystallography. Combining physics, chemistry, and mathematics, the structure of a molecule can be calculated based on experimental data. X-rays hitting atoms of a molecule will be redirected or diffracted. A layer of X-ray sensitive film changes color when X-rays are hitting the material. The resulting pattern caused by diffracted X-rays is based on the molecular structure, but in an indirect way. Experience and knowledge of physics, chemistry, and mathematics are needed to make sense of the data and figure out the structure of the molecule. Within two years, Rosalind Franklin not only set up a working lab, but she also came up with a brand new analysis of the structure of DNA. She prepared a scientific manuscript in 1953 and published it. She describes a double helix with phosphates at the outside. She also delayed her publication until she had done the tedious mathematical calculations that supported her model structure. There were no modern-day computers that could cut down calculations from weeks or months to mere days. Rosalind Franklin studied the structure of DNA and provided the exact calculations needed.
Her findings were taken without her consent and knowledge and used by Watson and Crick, who received the credit and also the Nobel prize. Anne Sayre’s book entitled Rosalind Franklin and DNA (published by Norton in New York in 1975) shines light on unethical or questionable behaviors. It was pointed out that Watson and Crick claim not to have known Rosalind Franklin’s data when they devised their model, but the facts discovered by others indicate the opposite, as pointed out on this website. It is also pointed out by some that Watson and Crick allegedly did not do any hands-on work with DNA in a “wet lab.” Their work was only theoretical, and they used the experimental findings of Rosalind Franklin without her consent. Rosalind Franklin’s story raises a glaring ethics controversy in gender bias.[3]
- Is it ethical to take someone else’s research findings without their consent?
- Is it ethical to take someone else’s research findings to improve your own research without acknowledging the contribution of that work?
- Is it ethical to treat someone with less respect or give them less credit because they are a female?

In Vitro Fertilization of Human Egg (1978 – “Test Tube Baby”)
When it seemed possible to fertilize a human egg in a test tube, ethical concerns arose.[4] It was considered unethical experimentation on human beings. Additional arguments were that the parents’ desire for a child should not prevail over a possibly unsafe method that could result in a child with severe health issues. In 1978, successful in vitro fertilization (IVF) occurred in England. Even though IVF is practiced today, ethical concerns are discussed.[5] IVF can help individuals in need. IVF can also raise ethical concerns. Ethical concerns are about the age limit of a mother for IVF, single women & same-sex couples, ownership of gametes & embryos, preimplantatory genetic testing, egg storage, egg sharing, surrogacy, commercialization, public funding, and religion. Some of the following questions were raised:
- Is it ethical for parents to have IVF at a high age when the parents will not live long enough to raise the child to adulthood?
- Is it ethical for a single woman to have a child through IVF?
- Is it ethical for same-sex couples to have a child through IVF?
- Is it ethical for woman to share some of her stored eggs with a female friend?
- Is it ethical for a person to offer eggs to others for profit reasons?
Cloning of Dolly the Sheep (1996)
In 1996, scientists succeeded in cloning an adult sheep using its somatic cells instead of its gametes (egg or sperm cells). The offspring generated from the cloning was named Dolly. Cloning has different meanings in different contexts. It can mean to make identical genetic copies of a part of a gene, of a whole gene, of a whole cell, or of a whole organism.[6] For Dolly, scientists removed DNA from the nucleus of the tissue of an adult sheep. Then that DNA was inserted into the egg cell of another sheep. The egg cell has genetic information within mitochondria from the donating mother. Therefore, Dolly was not 100% a clone. Dolly was euthanized at around the age of 5 years due to health complications. Sheep are culled in agriculture around 5 years of age. The maximum lifespan for sheep is estimated to be close to 23 years.[7] Dolly raised ethical questions about animal cloning. Some of them are listed as follows:
- Is it ethical to do cloning if at that time the success rate was at best 12%, and 88% was the lowest failure rate (rates from A. Fiester. 2005)?
- Is it ethical to expose the female animal to treatments and surgery to retrieve a useful egg for animal cloning?
- Is it ethical to expose the surrogate mother animal to treatments to bring the cloned egg to birth?
- Is it ethical to conduct animal cloning for a commercial purpose?
- Is it ethical to conduct animal cloning to preserve a species?
- Is cloning animals opening the door for attempts to clone humans?

Human Genome Project (1990–2003)
The Human Genome Project started officially in 1990 with the goal of sequencing all genes of the human genome, sequence all 3 billion base pairs that make up the entire human genome (non-coding and coding sequences), and study the ethical, legal, and societal issues (ELSI) of the project. The U.S. Department of Energy (DOE) and the National Institutes of Health (NIH) devoted 3% to 5% of their annual Human Genome Project (HGP) budgets toward studying the ethical, legal, and social issues (ELSI) surrounding availability of genetic information.[8] The Human Genome Project Information Archive lists the following areas and questions for ethical, legal, and social issues (ELSI):
Fairness in the use of genetic information by insurers, employers, courts, schools, adoption agencies, and the military, among others. Who should have access to personal genetic information, and how will it be used?
Privacy and confidentiality of genetic information. Who owns and controls genetic information?
Psychological impact and stigmatization due to an individual’s genetic differences. How does personal genetic information affect an individual and society’s perceptions of that individual? How does genomic information affect members of minority communities?
Reproductive issues including adequate informed consent for complex and potentially controversial procedures, use of genetic information in reproductive decision making, and reproductive rights. Do healthcare personnel properly counsel parents about the risks and limitations of genetic technology? How reliable and useful is fetal genetic testing? What are the larger societal issues raised by new reproductive technologies?
Clinical issues including the education of doctors and other health service providers, patients, and the general public in genetic capabilities, scientific limitations, and social risks, and implementation of standards and quality-control measures in testing procedures. How will genetic tests be evaluated and regulated for accuracy, reliability, and utility?
Uncertainties associated with gene tests for susceptibilities and complex conditions (e.g., heart disease) linked to multiple genes and gene-environment interactions. Should testing be performed when no treatment is available? Should parents have the right to have their minor children tested for adult-onset diseases? Are genetic tests reliable and interpretable by the medical community?
Conceptual and philosophical implications regarding human responsibility, free will vs genetic determinism, and concepts of health and disease. Do people’s genes make them behave in a particular way? Can people always control their behavior? What is considered acceptable diversity? Where is the line between medical treatment and enhancement?
Health and environmental issues concerning genetically modified foods (GM) and microbes. Are GM foods and other products safe to humans and the environment? How will these technologies affect developing nations’ dependence on the West?
Commercialization of products including property rights (patents, copyrights, and trade secrets) and accessibility of data and materials. Who owns genes and other pieces of DNA? Will patenting DNA sequences limit their accessibility and development into useful products?
An example is Huntington’s Disease. It raises questions about the psychological impact, clinical issues, and uncertainties. This disease is inherited, and often signs or symptoms show at the adult age of 30 to 40 years old. The damage to the nerve cells can impact movement, speech, thinking, and psyche. Unfortunately, the disease is progressive, and there is no cure. There is a psychological impact if someone finds out that they have a fatal debilitating disease. There are clinical issues. Doctors and the general public need to be educated about Huntington’s disease, about the consequences of finding out about a genetic disease, and about the fact that the disease’s severity has a range. The uncertainties include testing for an incurable disease such as Huntington’s disease. The disease’s severity ranges also between the number of trinucleotide repeats, but the demarcations are clear cut. The following ethical questions could be raised:
- Should testing be performed when no treatment is available?
- Should parents have the right to have their minor children tested for adult-onset diseases?
- Should an 18-year-old child tell their test results to their 35-year-old parent who does not want to get tested?
- Should an 18-year-old child tell their positive test result to a parent who doesn’t want to get tested and doesn’t want to know about their chances of having inherited that disease?
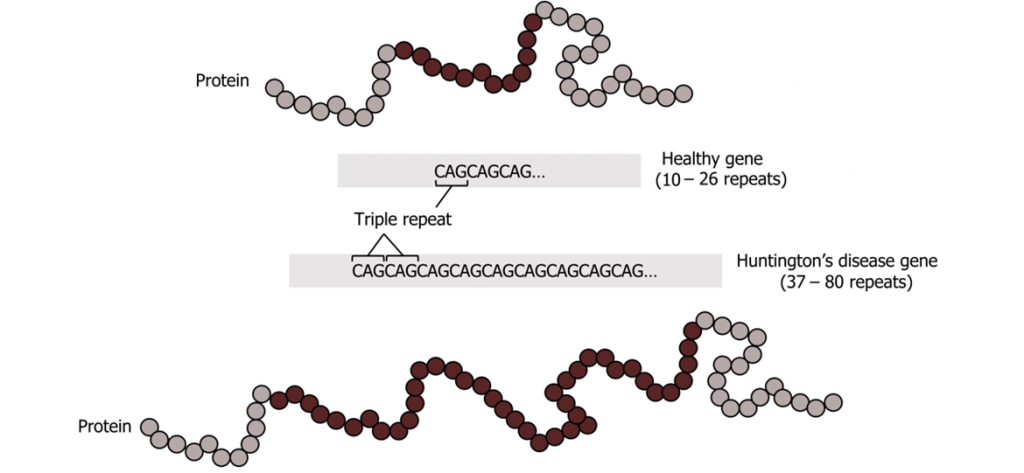
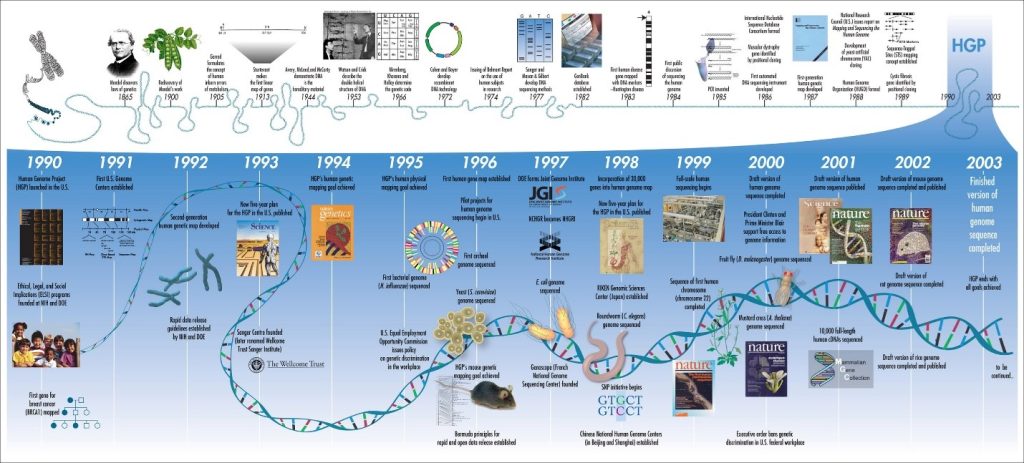
Figure 17.9: Timeline of genetic discoveries from genes by Gregor Mendel to the human genome project (HGP-in blue).
Ownership of a Gene (1990–2013)
In the USA, the human genome project raised also questions about ownership of genes and patent rights for genes. Scientists, institutions including the National Institutes of Health (NIH), and commercial entities were endeavoring to patent genes or genetic information.[9] Patents were granted for different reasons such as diagnostic uses, compositions of matter, and functional uses. The genes BRCA1 & BRCA2 were patented for diagnostic uses related to breast cancer and ovarian cancer. Insulin protein made from obtaining the gene sequence of the insulin gene falls in the category of patents for compositions of matter. An example of a functional use patent is a product or drug that is regulating a gene involved in making someone healthier or preventing disease. In 2013, the Supreme Court of the USA ruled that human genes themselves can’t be patented, but complementary DNA (cDNA) can. The patent claims by the company claiming broad patent infringements for the human BRCA1 & BRCA2 genes failed.
The following ethical questions can arise:
- If a research university is prevented from researching additional and deeper functions of a gene because research on the gene is prohibited by a gene patent, is that good, bad, neutral, and for whom?
- If patients can’t afford to be tested for a genetic disease because a gene patent requires expensive license fees for that test, is that good, bad, neutral, and for whom?
Further reading:
Merz, J. F., & Cho, M. K. (2005). What are gene patents and why are people worried about them?. Community genetics, 8(4), 203–208. https://doi.org/10.1159/000087956
Can genes be patented?: MedlinePlus Genetics. (n.d.). https://medlineplus.gov/genetics/understanding/testing/genepatents/
Supreme Court of the USA. ASSOCIATION FOR MOLECULAR PATHOLOGY ET AL. v. MYRIAD GENETICS, INC., ET AL. No. 12–398. 2013 free download at https://www.supremecourt.gov/opinions/12pdf/12-398_1b7d.pdf
Ownership of human genes. Correspondence: Richard M. Leibovitz. Robert Mullan Cook-Deegan Nature Vol. 382. page 17. 4 July 1996 https://www.nature.com/articles/382017a0.pdf?origin=ppub
Fiester, A. (2005). Ethical Issues in Animal Cloning. Retrieved from https://repository.upenn.edu/bioethics_papers/35 Reprinted with permission of Johns Hopkins University Press. The article originally appeared in Perspectives in Biology and Medicine, Volume 48, Issue 2, June 2005, pages 328-343. Publisher URL: http://muse.jhu.edu/journals/perspectives_in_biology_and_medicine/toc/pbm48.3.html
Harris J. “Goodbye Dolly?” The ethics of human cloning. Journal of Medical Ethics 1997;23:353-360. A version of this article is freely available as a pubmed central version using the link https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1377577/, accessed 3/24/2022
Barghachie, S. (2014, January 27). The hype about Dolly | Ethical Issues in Health Care. https://scholarblogs.emory.edu/philosophy316/2014/01/27/the-hype-about-dolly/
Authors: Mary Ann Clark, Matthew Douglas, Jung Choi. Publisher/website: OpenStax. Book title: Biology 2e. Publication date: Mar 28, 2018. Location: Houston, Texas. Book URL: https://openstax.org/books/biology-2e/pages/1-introduction Section URL: https://openstax.org/books/biology-2e/pages/17-1-biotechnology; accessed 3-24-2022
Review of the Ethical, Legal and Social Implications Research Program and related Activities (1990-1995). (n.d.). https://www.genome.gov/10001747/elsi-program-review-19901995
Human Genome Project Information. (n.d.). http://www.ornl.gov/hgmis
Murray, T. H. (1991). Ethical issues in human genome research. The FASEB Journal, 5(1), 55–60. https://doi.org/10.1096/fasebj.5.1.1825074
Huntington’s disease – Diagnosis and treatment – Mayo Clinic. (2022, May 17). https://www.mayoclinic.org/diseases-conditions/huntingtons-disease/diagnosis-treatment/drc-20356122
- Further reading is in this open access article Zanatta A, Zampieri F, Basso C, Thiene G. Galileo Galilei: Science vs. faith, Global Cardiology Science and Practice 2017:10 http://dx.doi.org/10.21542/gcsp.2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5871402/ ↵
- References: (1) Allhoff F. Evolutionary ethics from Darwin to Moore. Hist Philos Life Sci. 2003;25(1):51-79. doi: 10.1080/03919710312331272945. PMID: 15293515. https://pubmed.ncbi.nlm.nih.gov/15293515/ accessed 3/23/2022. (2) The difference of being human: Morality. Francisco J. Ayala. PNAS. May 5, 2010. 107 (supplement_2) 9015-9022 https://doi.org/10.1073/pnas.0914616107 accessed 3/23/2022). ↵
- There quite a few online resources available. Another one is https://onlineethics.org/cases/ethics-science-classroom/search-structure-dna accessed 3/23/2022. ↵
- Wymelenberg S; Institute of Medicine (US). Science and Babies: Private Decisions, Public Dilemmas. Washington (DC): National Academies Press (US); 1990. 7, New Technologies: The Ethical and Social Issues. Available from: https://www.ncbi.nlm.nih.gov/books/NBK235272/ accessed 3/23/2022 ↵
- Use of in vitro fertilization—ethical issues. Kjell Asplund. Ups J Med Sci. 2020; 125(2): 192–199. doi: 10.1080/03009734.2019.1684405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7721055/ accessed 3/23/2022 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/ ↵
- Reference Authors: Mary Ann Clark, Matthew Douglas, Jung Choi. Publisher/website: OpenStax. Book title: Biology 2e. Publication date: Mar 28, 2018. Location: Houston, Texas. Book URL: https://openstax.org/books/biology-2e/pages/1-introduction Section URL: https://openstax.org/books/biology-2e/pages/17-1-biotechnology; accessed 3-24-2022 ↵
- Hoffman JM, Valencak TG. A short life on the farm: aging and longevity in agricultural, large-bodied mammals. Geroscience. 2020;42(3):909-922. doi:10.1007/s11357-020-00190-4 ↵
- Reference Human Genome Project Information Archive. 1990–2003. http://www.ornl.gov/hgmis ↵
- Reference Merz JF, Cho MK. What are gene patents and why are people worried about them? Community Genet. 2005;8(4):203-208 ↵

