155 Prokaryotic Gene Regulation
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe the steps involved in prokaryotic gene regulation
- Explain the roles of activators, inducers, and repressors in gene regulation
The DNA of prokaryotes is organized into a circular chromosome, supercoiled within the nucleoid region of the cell cytoplasm. Proteins that are needed for a specific function, or that are involved in the same biochemical pathway, are encoded together in blocks called operons. For example, all of the genes needed to use lactose as an energy source are coded next to each other in the lactose (or lac) operon, and transcribed into a single mRNA.
In prokaryotic cells, there are three types of regulatory molecules that can affect the expression of operons: repressors, activators, and inducers. Repressors and activators are proteins produced in the cell. Both repressors and activators regulate gene expression by binding to specific DNA sites adjacent to the genes they control. In general, activators bind to the promoter site, while repressors bind to operator regions. Repressors prevent transcription of a gene in response to an external stimulus, whereas activators increase the transcription of a gene in response to an external stimulus. Inducers are small molecules that may be produced by the cell or that are in the cell’s environment. Inducers either activate or repress transcription depending on the needs of the cell and the availability of substrate.
The trp Operon: A Repressible Operon
Bacteria such as Escherichia coli need amino acids to survive, and are able to synthesize many of them. Tryptophan is one such amino acid that E. coli can either ingest from the environment or synthesize using enzymes that are encoded by five genes. These five genes are next to each other in what is called the tryptophan (trp) operon (Figure 16.4). The genes are transcribed into a single mRNA, which is then translated to produce all five enzymes. If tryptophan is present in the environment, then E. coli does not need to synthesize it and the trp operon is switched off. However, when tryptophan availability is low, the switch controlling the operon is turned on, the mRNA is transcribed, the enzyme proteins are translated, and tryptophan is synthesized.
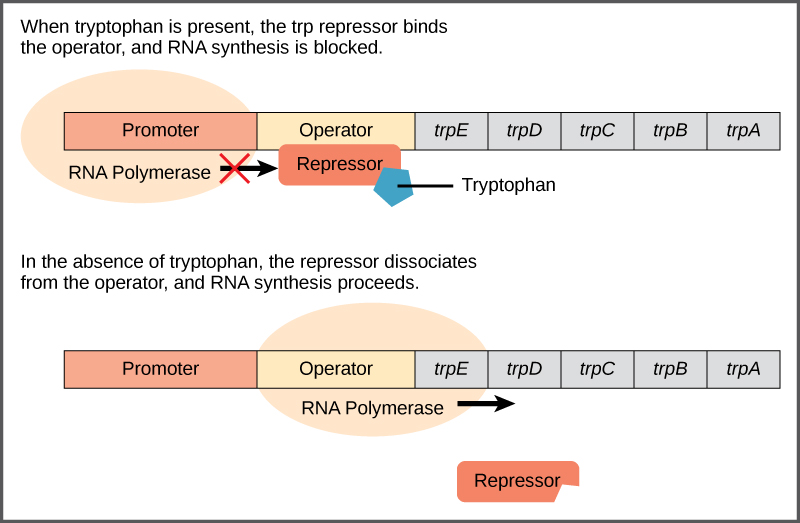
The trp operon includes three important regions: the coding region, the trp operator and the trp promoter. The coding region includes the genes for the five tryptophan biosynthesis enzymes. Just before the coding region is the transcriptional start site. The promoter sequence, to which RNA polymerase binds to initiate transcription, is before or “upstream” of the transcriptional start site. Between the promoter and the transcriptional start site is the operator region.
The trp operator contains the DNA code to which the trp repressor protein can bind. However, the repressor alone cannot bind to the operator. When tryptophan is present in the cell, two tryptophan molecules bind to the trp repressor, which changes the shape of the repressor protein to a form that can bind to the trp operator. Binding of the tryptophan–repressor complex at the operator physically prevents the RNA polymerase from binding to the promoter and transcribing the downstream genes.
When tryptophan is not present in the cell, the repressor by itself does not bind to the operator, the polymerase can transcribe the enzyme genes, and tryptophan is synthesized. Because the repressor protein actively binds to the operator to keep the genes turned off, the trp operon is said to be negatively regulated and the proteins that bind to the operator to silence trp expression are negative regulators.
Catabolite Activator Protein (CAP): A Transcriptional Activator
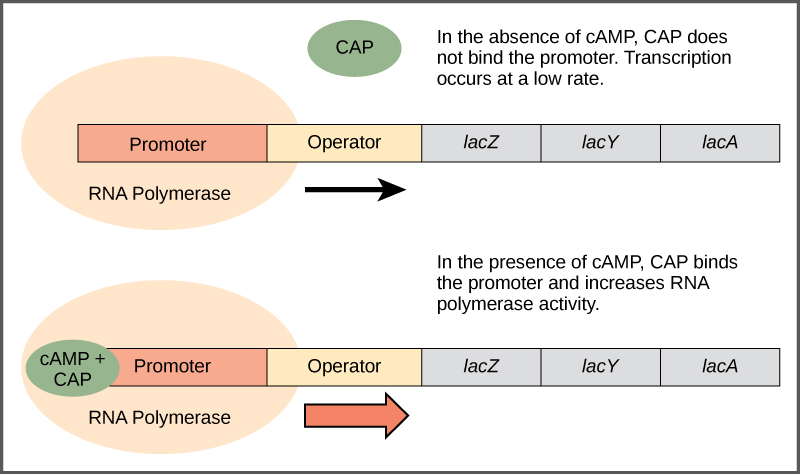
Just as the trp operon is negatively regulated by tryptophan molecules, there are proteins that bind to the promoter sequences that act as positive regulators to turn genes on and activate them. For example, when glucose is scarce, E. coli bacteria can turn to other sugar sources for fuel. To do this, new genes to process these alternate sugars must be transcribed. When glucose levels drop, cyclic AMP (cAMP) begins to accumulate in the cell. The cAMP molecule is a signaling molecule that is involved in glucose and energy metabolism in E. coli. Accumulating cAMP binds to the positive regulator catabolite activator protein (CAP), a protein that binds to the promoters of operons which control the processing of alternative sugars. When cAMP binds to CAP, the complex then binds to the promoter region of the genes that are needed to use the alternate sugar sources (Figure 16.5). In these operons, a CAP-binding site is located upstream of the RNA-polymerase-binding site in the promoter. CAP binding stabilizes the binding of RNA polymerase to the promoter region and increases transcription of the associated protein-coding genes.
The lac Operon: An Inducible Operon
The third type of gene regulation in prokaryotic cells occurs through inducible operons, which have proteins that bind to activate or repress transcription depending on the local environment and the needs of the cell. The lac operon is a typical inducible operon. As mentioned previously, E. coli is able to use other sugars as energy sources when glucose concentrations are low. One such sugar source is lactose. The lac operon encodes the genes necessary to acquire and process the lactose from the local environment. The Z gene of the lac operon encodes beta-galactosidase, which breaks lactose down to glucose and galactose.
However, for the lac operon to be activated, two conditions must be met. First, the level of glucose must be very low or non-existent. Second, lactose must be present. Only when glucose is absent and lactose is present will the lac operon be transcribed (Figure 16.6). In the absence of glucose, the binding of the CAP protein makes transcription of the lac operon more effective. When lactose is present, its metabolite, allolactose, binds to the lac repressor and changes its shape so that it cannot bind to the lac operator to prevent transcription. This combination of conditions makes sense for the cell, because it would be energetically wasteful to synthesize the enzymes to process lactose if glucose was plentiful or lactose was not available. It should be mentioned that the lac operon is transcribed at a very low rate even when glucose is present and lactose absent.
Visual Connection
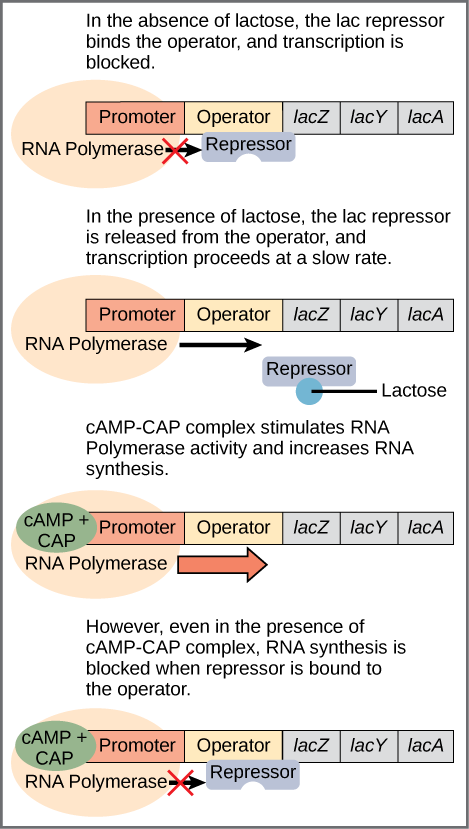
If glucose is present, then CAP fails to bind to the promoter sequence to activate transcription. If lactose is absent, then the repressor binds to the operator to prevent transcription. If either of these conditions is met, then transcription remains off. Only when glucose is absent and lactose is present is the lac operon transcribed (Table 16.2).
Signals that Induce or Repress Transcription of the lac Operon
| Glucose | CAP binds | Lactose | Repressor binds | Transcription |
|---|---|---|---|---|
| + | – | – | + | No |
| + | – | + | – | Some |
| – | + | – | + | No |
| – | + | + | – | Yes |
Link to Learning
Watch an animated tutorial about the workings of lac operon here.

