Comparing Rates of Fermentation in Yeast
Learning Objectives
After completing the lab, the student will be able to:
- Describe how different carbon compounds affect the rate of fermentation.
Activity 1: Pre-Assessment
- What is the difference between real sugar and a sugar substitute? Could either substance be used during cellular respiration?
- How could you tell that an organism, such as yeast, has switched from aerobic respiration to fermentation?
- Discuss the answers to questions 1 and 2 with the class.
Activity 1: Comparing Rates of Fermentation in Yeast
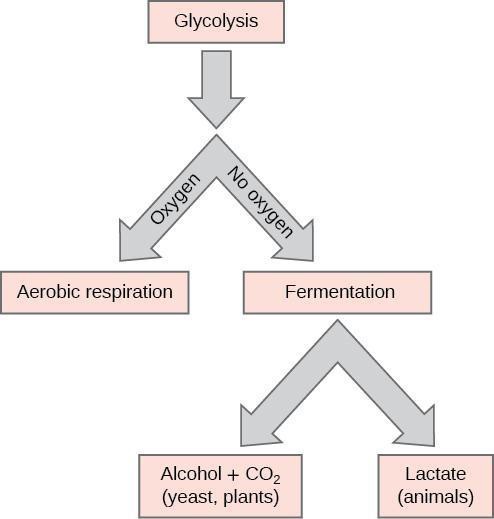
Yeast carry out fermentation as a means to access the chemical energy from their food, which, in this case, will be sugars such as glucose. In this activity, you will be comparing fermentation between these food sources and a control group of water. Yeast can exist in a state of dormancy, where they are alive, but their physical activity has temporarily stopped to minimize energy use. This adaptation allows these organisms to survive extended periods of drought and other harsh environmental conditions. As you add water to the dry yeast, you will be activating the cells out of dormancy, and they will resume physical activity and begin eating. Once the yeast absorbs the food molecule, it will first break the molecule down in a process called glycolysis. The word glycolysis literally translates into the breaking apart of glucose and this is the first step of the metabolism of sugars (Figure 9.1).
Shortly after mixing the activated yeast with the sugar molecules, fermentation will begin, and you will be able to observe gas bubbles being produced. This gas is carbon dioxide, one of the products of fermentation.
Safety Precautions
- Use care when using glassware.
- Be careful when inverting the graduated cylinder into the Petri dish.
- Inform your teacher immediately of any broken or cracked glassware, as it could cause injuries.
- Clean up any spilled liquids to prevent people from slipping.
For this activity, you will need the following:
- Graduated cylinder (50 ml)
- Petri dishes
- Beaker/container for yeast solution (150–200 ml)
- One package of dry yeast
- Warm water
- Room temperature water
- Glucose
- A sugar substitute
- Pipette or droppers
- Paraffin wax paper
- Paper towels
For this activity, you will work in pairs.
Structured Inquiry
Step 1: Add the yeast packet to the beaker and add 100 ml of warm water to activate it. Swirl slightly until water and yeast are mixed. Allow 10 minutes for activation. During this time, create a data table to measure the volume in milliliters of the carbon dioxide gas bubbles as a function of time. Include space for two trials for each variable of water, glucose, and the sugar substitute, as well as predictions of the amount of respiration that will occur for each sugar.
Step 2: Hypothesize/Predict: Based on what you know about the differences between water, glucose, and the sugar substitute, predict how much carbon dioxide concentrations will differ in the presence of 1) glucose, 2) the sugar substitute, and 3) water. Add your predictions to the data table created in Step 1.
Step 3: Student-Led Planning: You will now start with the first trial of measuring carbon dioxide gas bubbles produced from fermentation. Choose a time interval to take down measurements. After the first trial, adjust the time intervals if necessary for taking down measurements. These should be in your data table. Determine and record the quantity of glucose and sugar substitute that you will use, as you should perform more than one trial of each test.
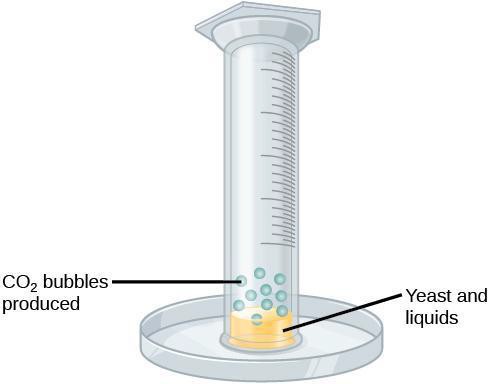
Step 4: Pour 15 ml of yeast solution into the graduated cylinder. Then add in the water and fill up the rest of the cylinder. For the other trials, glucose or sugar substitute will be added instead of water. As soon as you add the water, cover the cylinder with paraffin wax paper and invert it, to avoid spilling the fluid. One person can hold the graduated cylinder in place displacing it carefully and completely onto the Petri dish, as shown in Figure 9.2. As fermentation takes place, bubbles will rise into the cylinder, and the volume of the bubbles can be recorded. To do this, you will read the measurement markings at either end of the bubbles to get at total volume reading.
Step 5: Student-Led Planning: Discuss with your partner how the first trial worked and whether any adjustments need to be made to the set-up to ensure more accurate results. If the original trial did not go well due to measurement issues, repeat after adjusting the procedure. Then use the corrected trial as the first one. All subsequent trials will then use the same exact procedure.
Step 6: Critical Analysis: Record the amount of carbon dioxide gas produced in your data table. Are the predictions made in step 2 supported by the data? Why or why not? What is the control in this experiment? Is it a positive or negative control and why? What changes to the quantities of liquids, time period, or set up can you make to improve your results? Discuss with your partner and write your answers in your notebook.
Guided Inquiry
Step 1: Hypothesize/Predict: How do you think the chemical makeup of the compound added to the yeast solution affects the rate of fermentation? How different do you think the results will be with the glucose and sugar substitute trials? Write your hypotheses in your notebook.
Step 2: Student-Led Planning: Make any adjustments to your first trial set up and re-do if necessary. Then, carry out the second trial for water and two additional trials each using glucose and sugar substitute. Record your measurements in the data table.
Step 3: Critical Analysis: Which compound led to the highest rate of fermentation? Was there a significant difference between the glucose and sugar substitute trials? Discuss your answers with your partner and write them in your notebook.
Assessments
- What does data from the experiment show about the energy needs of yeast?
- What do you think would happen to the activated yeast if no carbon-based molecules were provided?
- What environmental reasons would cause yeast to go dormant?

