Dependence of Photosynthesis on Selected Wavelength in the Light Spectrum
Learning Objectives
After completing the lab, the student will be able to:
- Analyze the dependence of O2 production on the color of light spectrum and other environmental variables.
Activity 2: Pre-Assessment
- Answer the following questions in your notebook: Spirogyra are thin, thread-like green algae that cluster up in rafts and float on the surface of ponds buoyed by bubbles of gas. What gas buoys the network of algae? What happens when there is no light?
- Answer the following questions in your notebook: If chlorophyll absorbs maximally in the blue and red regions of the visible spectrum and carotenoids extend absorbance in the blue-green region, which color of irradiation will be the most effective: red, green, or white?
- Discuss the answers to questions 1 and 2 with the class.
Activity 2: Dependence of Photosynthesis on Selected Wavelength in the Light Spectrum
Several environmental factors influence the light-dependent reaction of photosynthesis. In this activity, you will investigate the rate of the light-dependent reaction by measuring indirectly the production of oxygen. Leaf disks are suspended in a solution of bicarbonate. A small drop of dishwashing soap in the solution breaks the surface tension of the leaf disks and allows the bicarbonate to penetrate inside the leaf. The bicarbonate in the solution provides a source of CO2 for photosynthesis. The inner spaces in a leaf are normally filled with air. When a bicarbonate solution is forced into those air spaces, the leaf disks become heavier and sink to the bottom of the solution.
As photosynthesis proceeds, the oxygen produced by Photosystem II (PSII) pushes liquid out of the air spaces and eventually bubbles out of the disks, which float to the surface. By monitoring the time it takes the disks to float, the rate of photosynthesis can be estimated under various conditions. To compare results of experiments, you will use the estimated time for 50 percent of the disks to float to the surface, labeled as ET50. This is a median value that allows you to discount outliers, such as a floating disk that never sank in the first place or a disk stuck to the bottom of the cup. Because ET50 values decrease when photosynthetic rates go up, you may choose to plot 1/ET50 as a function of your independent variable.
Safety Precautions
- Wear goggles and an apron
- Use caution while working with the lamp, as light bulbs become very hot
- Use caution while handling glass
For this activity, you will need the following:
- Plant material: intact leaves of spinach and Coleus, and light-colored leaves such as iceberg lettuce, celery leaves, or cabbage (one leaf per pair of students). Avoid fuzzy leaves that trap air on their surface and do not sink easily.
- 0.2 M sodium bicarbonate solution (0.4 M, 0.6 M, and 0.8 M should be available)
- Two to three plastic 10 mL syringes
- Clear plastic cups
- Dilute dishwashing soap solution
- Single-hole punch
- Lamps
- Acetate colored filters (at a minimum, use blue, red, and green filters)
- Aluminum foil
For this activity, you will work in pairs.
Structured Inquiry
Step 1: Hypothesize/Predict: Discuss with your partner how different the wavelengths of light would affect the rate of photosynthesis. Write in your lab notebook the justification for your predictions. Include the controls you will need to run a meaningful experiment.
Step 2: Student-led planning: In this guided inquiry, you will measure the ET50 in white light, in the dark, and in different regions of the visible spectrum. Design your experiment so you change only one variable at a time. Can you devise a set-up to minimize stray white light when you test the effect of colored light? Some experimentalists build a cardboard box with an opening that can be covered by a filter. In this case, run all your experiments under the same conditions using a transparent filter for white light and aluminum foil to block the light. Create a table for your data.
Step 3: Set up of experiment:
- Using a single-hole punch, punch 10-15 disks from an intact leaf being careful not to damage the leaf in the process.
- Transfer the disks to the barrel of a 10 mL syringe and replace the plunger.
- Pull about 4–5 mL suspension solution in the syringe. Tapping gently on the barrel until all disks float freely
- Push the plunger to expel excess air.
- Create a vacuum in the syringe by holding your index finger on the tip of the syringe where the needle would be inserted and slowly draw the plunger down. You will feel the vacuum pulling on your finger. Do not draw the barrel out of the syringe. The solution should be flowing into the airspaces of the leaf disks. Wait two to three minutes and release the vacuum. The disks should sink after you repeat this procedure two to three times. See Figure 11.3.
- Tap the syringe gently to re-suspend the disks and empty the contents of the syringe into a clear cup. Cover the disks with additional solution if needed to submerge them.
- Continue with your experimental set-up. Illuminate the clear cups with the lamp and record the number of floating disks every minute for 20 minutes. For the dark control, check the disks after 20 minutes.
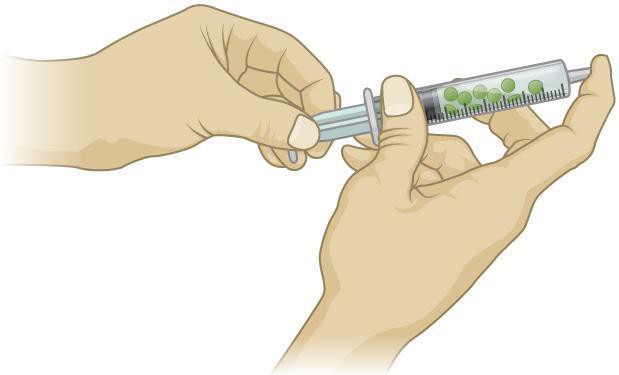
Figure 11.3: Pulling the barrel of the syringe and capping the tip with a finger creates a vacuum inside the syringe.
Step 4: Critical analysis: Plot the number of floating disks for each condition as a function of time and determine the ET50 for each condition. Compare the estimated photosynthetic rates as 1/ET50 at the wavelengths you tested. Do the results confirm your hypothesis? Plot your results in a graph.
Guided Inquiry
Step 1: Hypothesize/Predict: Discuss with your partner which conditions would impact the rate of photosynthesis in the disk. Conditions include the following:
- The effect of varying CO2 concentration. This is achieved by using different concentrations of a sodium bicarbonate buffer that releases CO2.
- Plant material differences, such as comparing the deep green leaves of spinach to the pale leaves of cabbage or celery
- Light intensity differences, such as by changing the distance between the light source and the cup
Recall that I=1/D2, where I is the intensity of light and D is the distance between the light source and the target. You can use different areas of a variegated leaf from Coleus.
Choose a single variable you will study in your experiment. Write down in your notebook the justification for your experiment and your prediction. How will it improve your understanding of photosynthesis? Include the controls you will need to run a meaningful experiment. Once you have determined a condition, discuss the feasibility of the experiment with your instructor and be prepared to modify your experiment. After choosing a condition, design your experiment carefully to limit the effect of a single variable.
Step 2: Student-led planning: Sketch your set-up in detail and ask your teacher for approval. Modify your set-up per the feedback from your teacher if need be. Now you are ready to assemble all the materials that you will need to perform the experiment. Follow the instructions described in Step 3 under the structured inquiry. Count the number of disks that float over time to estimate the ET50. Enter all data in a table like Table 11_02, which you will adapt to your experimental conditions.
Step 3: Critical analysis: Plot the number of floating disks for each experimental condition as a function of time and determine the ET50 for each condition. Plot the estimated photosynthetic rates using 1/ET50 as a function of your independent variable, either bicarbonate concentration or light intensity. Compare the 1/ET50 values for darkly pigmented leaves and pale leaves. In each case, discuss the validity of the data and how you would improve on the experiment. How reliable is it to use bicarbonate as a source of CO2? Can you compare light intensities without actually measuring light flux? Is it a fair comparison to use the response of disks from different types of plants? What else could have been at play?
Assessments
- In an experiment, water is labeled with the radioactive O18 isotope. The labeled water is then supplied to the leaf disks. In a second experiment, CO2 labeled with the isotope O is supplied to the disks. In which of the two experiments will you detect O18 in the product of photosynthesis? Why is this so?
- DCMU (3-(3, 4-dichlorophenyl)-1, 1-dimethyl urea) is an herbicide that specifically inhibits transfer of electrons from PSII. What would you predict would happen to the production of oxygen in an Elodea twig submerged in a solution treated with DCMU? How would an animal such as a water snail be affected by the presence of DCMU in the same environment? You can assume that DCMU doesn’t affect the snail directly.
- What would happen to gas bubbles in Elodea if a test tube is exposed to light for a prolonged period without aeration? What would happen if water snails were added to the test tube? Illustrate your answer with a diagram showing gas the interaction between the Elodea and the water snail.
Extension Activities
- Measure the ET50 of disks from plants maintained overnight in the light and in the dark. Do plants maintained in the dark perform photosynthesis faster, slower, or at the same rate? Write your prediction with an explanation and discuss your results.
- Compare ET50 at different temperatures, such as 10°C, room temperature, and 50°C. Which steps of photosynthesis may be temperature dependent and how do they affect the evolution of O2?

