Measurement of Respiration and Effect of Temperature
Learning Objectives
After completing the lab, the student will be able to:
- Measure the consumption of oxygen during respiration.
- Measure the effect of environmental conditions on respiration in pea seeds.
Activity 2: Pre-Assessment
- Students stain corn seeds over a period of several days after the seeds are soaked with water to promote germination with iodine. Iodine stains starch blue. The students observe that the amount of starch decreases during germination. Can you explain this observation? Which metabolic process uses up starch?
- What kind of biological catalysts are involved in the reactions of respiration? If the rate of a chemical reaction doubles with the temperature, would you expect that rates of respiration to increase continuously with temperature?
- Discuss the answers to questions 1 and 2 with the class.
Activity 2: Measurement of Respiration and Effect of Temperature on Respiration Rate
Imagine that you plan to monitor respiration in a whole organism, such as a small invertebrate or a seedling. You may decide to follow the disappearance of the reactants, either glucose or oxygen. Your second choice is to measure the formation of the products, either water or carbon dioxide. In this laboratory, you will design experiments to assess the effect of environmental conditions on the process of cellular respiration.
In respiration, oxygen is consumed and CO2 is released. In this experiment, we will measure the disappearance of oxygen. A respirometer consists of an enclosed chamber in which the studied organism is placed and a graduated pipette with which we measure changes in the gas volumes. The CO2 gas that forms will be removed by adding Ca(OH)2, which reacts with carbon dioxide to form the insoluble salt CaCO3, calcium carbonate.
While measuring the changes in the amount of gas produced, you will consider the ideal gas law equation which can be stated as
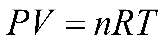
P represents the atmospheric pressure in mmHg, V is the volume of the gas in liters, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in degrees Kelvin. In the respirometer, pressure remains constant as the gas produced displaces water in the tube. We will set up the respirometers in a water bath to minimize fluctuations in temperature.
In this experiment, you will use pea seeds. In a seed, like the yellow peas shown in Figure 8.2, a tough coat protects the plant embryo. Nutrients in the form of starch and lipids surround the embryo and support its germination, or growth from seed, until the appearance of photosynthetic structures. Seeds are normally dormant, that is metabolically inactive, until the environmental conditions helpful for growth are available. In order to bring the seeds to an active state, (out of dormancy), the seeds you will use were soaked in water via a process called imbibition, for 6 to 8 days.

Cellular respiration involves three major sequential stages: glycolysis, the citric acid cycle, and oxidative phosphorylation. Oxygen serves as a terminal electron acceptor. Glycolysis takes place in the cytoplasm whereas mitochondria are the site of the citric acid cycle and the electron transport chain.
All the steps of respiration are mediated by enzymes, biological catalysts—mainly proteins—that lower the activation energy, the energy required to be available in a system before a chemical reaction can take place. Enzymes are not used up by the reactions they catalyzed. The process of respiration responds to the same environmental factors that affect the activity of enzymes. In this activity, you will measure the effect of temperature on respiration rates.
Safety Precautions
- Handle test tubes or glass containers with care; insert the plug by holding the container in a paper towel.
- Use plastic pipettes rather than glass pipettes.
- Wear goggles or safety glasses.
- Wear gloves when working with KOH or lime [Ca(OH)2], which are corrosive chemical compounds.
- Use care while handling hot water. Wear mitts and do not leave boiling water or a hot plate unattended.
- Protect your clothes with an apron.
- Inform your teacher immediately of any broken glassware as it could cause injuries.
- Clean up any spilled fluids to prevent other people from slipping.
- Wash your hands with soap and water after completion of the activity.
For this activity, you will need the following:
- Dried yellow peas
- Glass beads
- Balance and weigh boats
- Paper towels to imbibe seeds
- KOH or lime water
- Food coloring
- Absorbent and non-absorbent cotton
- Drilled rubber stoppers that fit the opening of the test tubes or bottles
- 1-ml plastic pipettes
- Top loading balance
- Thermometers
- Water baths
- Weights such as clamps or hex keys
- Wide glass test tubes or bottles
- Stirring rod
- Ice
- Hot plate to boil water
- Mitts
For this activity, you will work in pairs.
Structured Inquiry
Step 1: Obtain 25-30 germinating peas, dry peas, and glass beads to start your experiment. Place the germinating peas in a weigh boat and measure their weight. Record the weight in your notebook and then repeat for the dried peas and glass beads.
Step 2: In this activity, you will indirectly measure the rate of respiration of the peas by monitoring the decrease in gas when the peas are placed in the respirometer chamber. What gas will decrease in the chamber as the peas undergo respiration? Hypothesize how much the gas levels will likely change for the germinating seeds, dry seeds, and glass beads. Record your hypotheses and predictions in your notebook.
Step 3: Student-Led Planning: Which of your treatments serve as a control? Is this a positive or negative control? How will this control reveal whether or not the experiment is functioning properly? Write your answers in your notebook.
Step 4: Assemble a respirometer using Figure 8.3 as a guide and following the steps below.
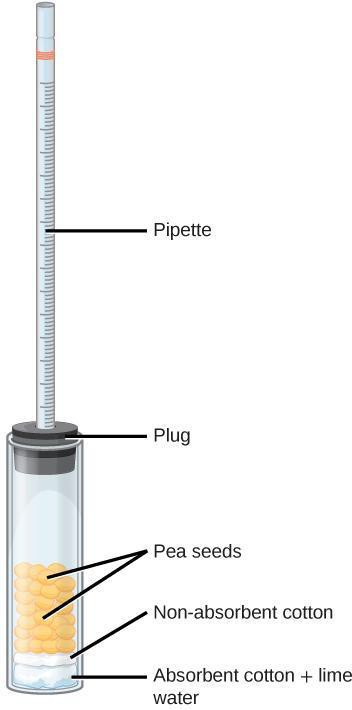
- In a wide test tube (or bottle), drop a pad of absorbent cotton. Pack down the cotton with a stirring rod. Add lime water Ca(OH)2, being careful not to oversaturate the pad or drip the lime water on the side of the tube.
- Insert a thin layer of non-absorbent cotton, pushing down with the glass rod. The cotton protects the seeds from lime water; however, if it is too thick, it will interfere with the diffusion of CO2.
- Plug the test tube with a bored rubber stopper. Add a drop of colored water in a 1-ml graduated pipette and insert the pipette in the hole of the stopper. Adjust the position of the drop by inserting a syringe in the stopper until you can easily read the position of the dye. (The syringe is not shown in Figure 8.3.) Rub some petroleum jelly where the pipette comes into contact with the rubber stopper. The respirometer must be water tight to yield reliable results. It is also possible to wrap the openings with stretchable plastic film.
- You may want to test for leaks by immersing the respirometer with the plug and pipette before filling it with reagents and cotton.
Step 5: Assemble the respirometer containing the control sample in the same manner.
Step 6: Immerse the respirometers with the experimental sample and the control in the water bath. Lining the water bath with a white paper towel will make it easier to read the markings on the pipettes. Make sure that the pipettes are resting across a piece of ribbon or string that spans the width of the water bath, as illustrated in Figure 8.4. The goal is to keep the pipettes out of the water while the test tubes remain submerged.
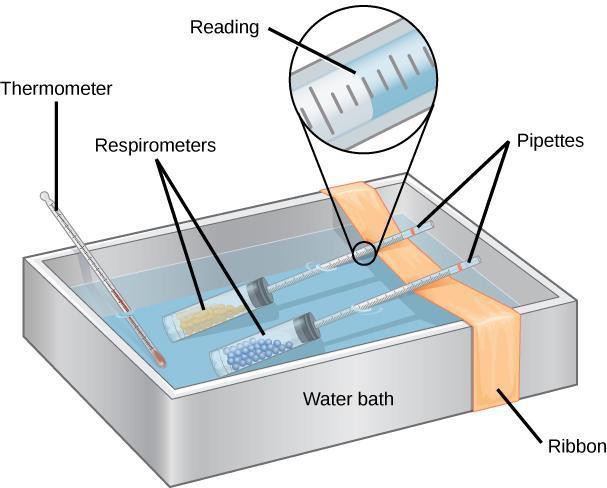
Step 7: Let the respirometers equilibrate for 5–10 minutes.
Step 8: Read the starting volume on the pipette. This is time 0 min. Record the displacement of the colored bead for all samples every 2 minutes for 20 minutes and enter data in a table of measurements.
Step 9: Critical Analysis: Calculate the changes in volume where the reading at time 0 is subtracted from every subsequent reading. Subtract the rate of volume change measured in the control samples to obtain a corrected rate of respiration.
Graph the changes in volume in respirometers as a function of time and calculate the rate of change from the slopes of the line plots. Calculate the rate of change per gram of seed. This will allow you to compare values obtained from different samples. Draw a plot of changes in gas volumes from the data in your table. What measurements will you enter on the axis? What measurements will you enter in the y-axis? Determine the rate of respiration in your experiment. How did you use the data from your control or controls? Did volumes change during the experiment? Which gas caused the change in volume? Do the results support your hypothesis? Can you explain unexpected results? Were the respirometers water-tight at all times? How could you modify the experiment in the future? Write your answers in your notebook.
Guided Inquiry
Step 1: Repeat the steps to set up the respirometers described in the Activity 2 Structured Inquiry. Use three water baths at the following temperatures: 10°C, room temperature (see Structured Inquiry), and 50°C.
Step 2: Hypothesize/Predict: Discuss with your partner what kind of influence temperature might have on metabolic processes. How would the respiration rate measured at 10°C compare to the rate measured at room temperature? Will the rate of respiration be higher at 30°C than room temperature? Do you predict that the rate of respiration will be higher at 50°C than at room temperature or 30°C? Enter your hypotheses in your notebook.
Step 3: Student-Led Planning: You will now measure the rate of respiration at three different temperatures. Discuss with your partner if you need to run the experiment at room temperature again. Decide which control you will set up for this experiment. Make a note of all the steps you will perform, as you did in Activity 2, and create tables for your observations in your lab notebook. You will take readings of the colored water bubble at 2-minute intervals for 20 minutes. Have your teacher approve your experimental procedure before proceeding.
Step 4: Once approved, carry out your experimental procedure, closely monitoring the temperature as you take measurements.
Step 5: Critical Analysis: Graph the changes in gas volumes from the data in your table for all three temperatures for the experimental and control set-up, as you did for the Structured Inquiry. Determine the rate of respiration for each temperature. Because the gas law shows that differences in temperature affect volumes, you must correct for any changes in volume that are a consequence of temperature variations rather than respiration. To do this, subtract changes in volumes measured in the respirometer containing glass beads from the changes in volume measured in the tubes containing germinating seeds held at the same temperature. Do the results support your hypothesis? Explain whether your results support or refute your hypothesis. How could you modify the experiment in the future? Write your ideas in your notebook.
Assessments
- Students record changes in gas released from respirometers containing germinating seeds and dry seeds. They set up their tubes in air rather than in a water bath. A thermometer probe is inserted in each respirometer. The tube that contains germinating seeds shows an increase in temperature. No such increase is recorded in a respirometer that contains dry seeds. What is the reason for the difference in temperature?
- The ideal gas law shows that volume depends on temperature as well as pressure. Why do you set your respirometers in a water bath?
- A classmate insists that there are no mitochondria in leaves because chloroplasts produce ATP through photosynthesis. How would you experimentally disprove this claim?

